EuropaBio lobbies for better Orphan Drug rules
EuropaBio has responded to the European Commission's public consultation on the Orphan Medicinal Products (OMPs) and Paediatrics Regulations.
The Orphan Drug Regulation adopted in 2000 should be improved to incentivise even ultra-rare conditions with high unmet medical need, according to EuropaBio, the EU bioindustry association. While the current Regulation has boosted the number of applications for orphan drug designation to 3,678 by the end of 2020, some issues still remain.
According to estimates 80% of rare disease patient population suffer from only 4% (390) of the known rare conditions. While most orphan drugs developed so far address conditions, such as molecularly defined cancer indications, affecting these 80% of patients, this still leaves a significant number of patients lacking sufficient treatment options, particularly those with conditions which can be considered ultra-rare. Novel solutions could further encourage development for the 95% of rare diseases with little or no available treatment. EuropaBio’s statement says. To do this, access pathways should provide more saftey as to when and where treatments will actually reach patients. Otherwise R&D and orphan drug commercialisation could be discouraged.
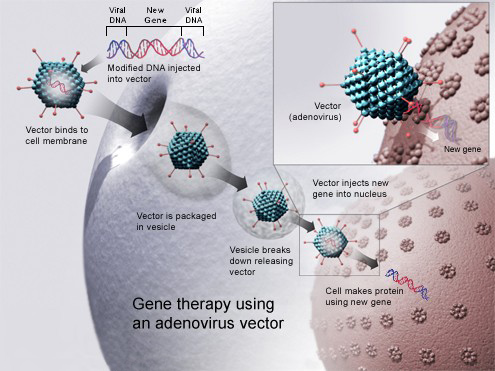
An example is bluebird bio, which withdrew its gene therapiy Zynteglo (betibeglogene autotemcel) for transfusion-dependent ?-thalassemia (TDT) from the German market after reimbursement negotiations failed. Meanwhile, the company stalled activities in the EU.
EuropaBio has suggested several options worth exploring e.g., a new model for public-private partnerships, building upon the European Reference networks, and utilising the upcoming European Health Data Space for rare disease research.



 Unsplash+
Unsplash+