CAR-Ts: Novartis wins UK
The NHS England announces it will reimburse Novartis' CAR-T cell therapy Kymriah as treatment for children with B cell acute lymphoblastic leukemia (ALL) that are refractory, in relapse post-transplant or in second or later relapse.
The European Commission approved Kymriah last week for the treatment of children with the rare blood cancer ALL as well as for adults with refractory diffuse large B cell lymphoma (DLBCL). According to a NICE spokesman, the NHS will pay a sum beyond Kymriah’s list price of £282,000 per patient. However, as the treatment often requires immunosuppression with IL-6 antibodies due to cytokine release syndrome, overall treatment costs will be significantly higher. The agreement is a bit symbolic as only 15 to 20 children in the UK with acute lymphoblastic leukaemia (ALL) are expected to be eligible for the treatment.
The real test case for reimbursement of CAR-T cell therapies will be the DLBCL indication, which affects about 4.600 patients in the UK, 200 thereof eglible to receive a CAR-T cell therapy. Novartis is still seeking an agreement with NHS for adults with DLBCL who got relapses even after two treatment cycles with different chemotherapeutics. Novartis’ EU competitor Gilead Sciences, whose CAR-T cell therapy Yescarta (axicabtagene ciloleucel) has been approved on the same day as Kymriah for DLBCL, has been already been rebuffed as "not cost-effective" by the UK’s NICE.
The NICE argued that that the drug’s incremental cost-effectiveness ratio more than doubled the threshold of £50,000 per quality-adjusted life year (QALY) gained compared with standard of care. What’s worse for Gilead Sciences, which wanted to roll-out Yescarta in Germany, Austria, France and the UK, is that its CAR-T cell therapy did not meet the criteria required for coverage by the Cancer Drugs Fund, which provides patients access to cancer drugs that would not otherwise be available from the NHS. Marketing of CAR-T therapies in Europe will be a head-to-head race between Novartis and Gilead Sciences.
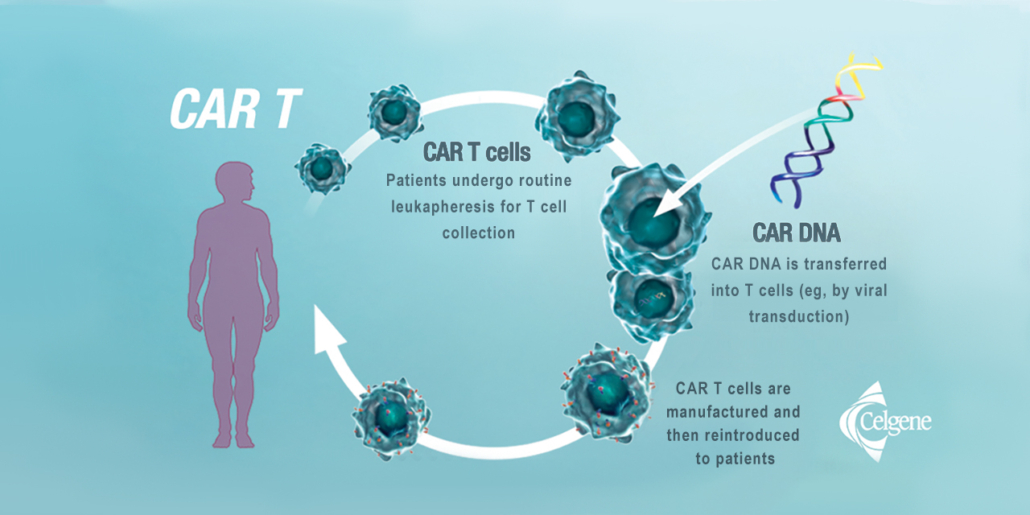
Price debates in the US have preceded the first reimbursement agreement for a CAR-T therapy in Europe. The high treatment price is attributed by the companies to the high production costs of cell therapies. Experts told European Biotechnology, that the total production cost for Kymriah including personal costs, logistics etc should be somewhat over US$100,000 as every one-time treatment with autologous CAR-T cells must be produced individually to get enough cells with chimeric antibody receptor that activates T cells to fight blood cancer cells. Pure material costs are estimated to be in the range of US$20,000 as confirmed by Novartis licensor Carl June.




