Argenx antibody kills AML
Argenx SE announced it achieved an overall response rate of 92% in patients with AML with its anti-TNFSF7 (CD70) antibody cusatuzumab.
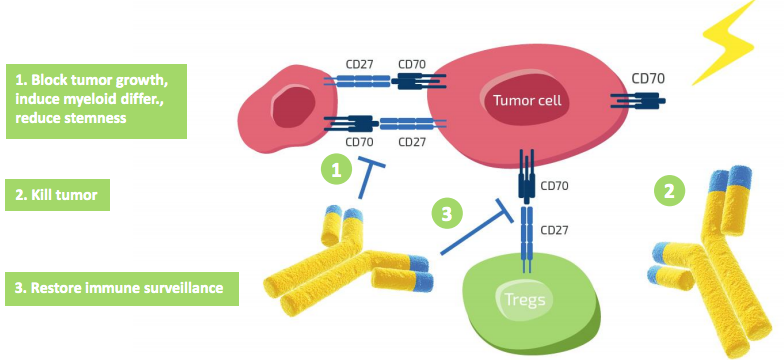
Overall, 11 of 12 patients with acute myeloid leukemia (AML) enrolled within a Phase I dose finding study, responded to the TNFSF7 blocker in combination with azacitidine, 9 of them showed a complete response. TNFSF7 is a cytokine that is overexpressed in AML myeloblasts. Cusatuzumab (ARGX-110) acts in trifold manner: it inhibits tumour cell proliferation, eliminates tumour cells and prevents tumour immune escape by blocking the activation of immune-dampening regulatory T cells (Tregs). In preclinical tests, the llama-derived defucosylated IgG1 antibody showed strong complement-dependent cytotoxicity and antibody-dependent cellular phagocytosis activity. Furthermore, cusatuzumab blocked activation and proliferation of Tregs by neutralising TNFSF7, which activates that process through binding to the CD27 receptor.
However, as with most blood cancer therapies, response didn’t last long (6.9 months). However, the company said that 5 patients showed no signs of mimimal residual disease.
In Phase I trials on 94 patients in other cancer indications, cusatuzumab was previously shown to have an excellent safety profile. Detailled results will be presented at the beginning of December at the meetin of the American Society of Hematology (ASH).


 Brainomix
Brainomix BioNTech SE
BioNTech SE