International control of hemp and cannabinoids
For a plant that has been used for millennia, controls on the supply of psychoactive and non-psychoactive components extracted from cannabis are relatively recent, with global regulations starting in the 20th century with the International Opium Conventions. The current, and often conflicting, regulatory landscape has focused on definitions of hemp and cannabidiol (CBD). Reference materials are critical to defining the % ?9-THC in these products.
The earliest recorded attempt to control cannabis was 1378, when Soudoun Sheikouni, the emir of the Joneima in Arabia, prohibited cannabis use. He instructed that all cannabis plants in the region be destroyed, and users were punished by having their teeth extracted. In the following centuries, controls were introduced by several individual countries with little international coordination until the International Opium Conventions. The current formal controls that now apply originate from the United Nations 1961 Single Convention on Narcotic Drugs. Cannabis and its resin were placed under Schedules I and IV, with ?9-THC (and selected stereoisomers) being controlled via Schedule I of the United Nations 1971 Convention on Psychotropic Substances. This is the primary legislation that is in force globally. However, in 2019, the World Health Organization (WHO) recommended amending the entry for cannabis and its resin to exclude preparations of CBD that are not more than 0.2% ?9-THC.
Defining hemp and CBD
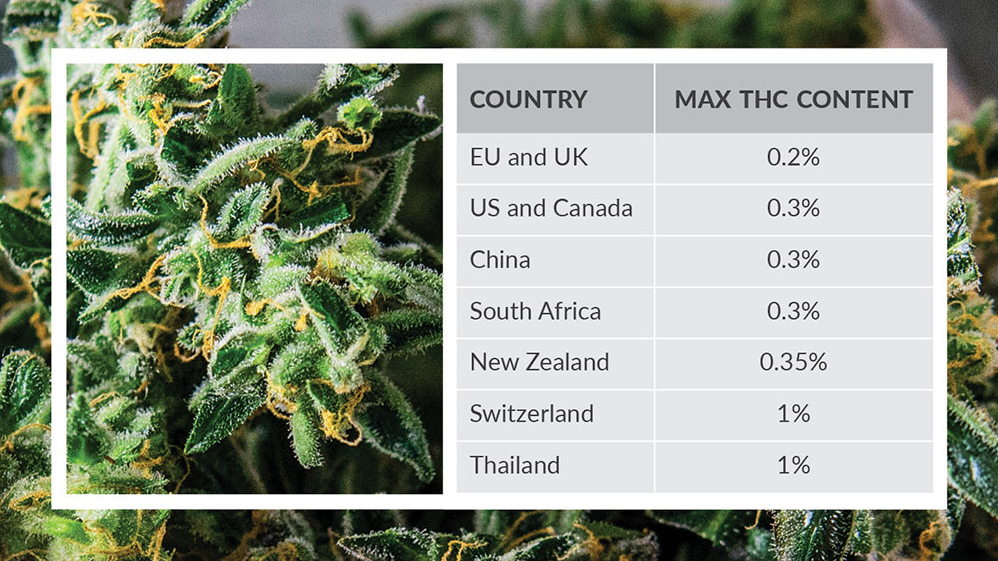
Any such enacted legislation needs to take into consideration the plant material, extracts, and oils, as well as the individual active and inactive compounds from cannabis. Complicating matters, international and country-specific drug laws often have conflicting guidelines that could have significant repercussions on individuals or organizations if a particular product or batch falls outside legal or regulatory definitions. The current lack of international consistency in the definition of industrial hemp or CBD products surrounds the percentage of controlled cannabinoids permitted to be present. The figure lists the % of ?9-THC allowed in the plant dry weight that legally defines a product as hemp and not subject to regulatory control. These limits generally have little flexibility. A similar approach to products containing CBD has been recommended in the UK by the Government Chemist guidance.
Defining the % of ?9-THC in cannabis products requires reliable and effective analysis of representative samples to determine the true value of any target analytes. There is no internationally agreed upon standard methodology for the measurement of cannabinoids in the way there are clearly defined pharmacopeial methods, so every laboratory has its own varying analytical processes. This can give rise to differences in results between laboratories. One approach to improve robustness is to employ an accredited testing laboratory that uses accurately prepared calibration standards and reference materials. ISO 17034-manufactured reference materials will improve analytical reliability in combination with robust analytical methodology. These materials can be single components that are combined into a mixture by the end-user or formulated as a premade Certified Reference Material mixture. Premade mixtures are typically more cost-effective and increase precision and accuracy compared to single solutions, which provides analytical data confidence and mitigates regulatory-related risks.
This article was originally published in Summer Edition European Biotechnology Magazine 2021.


 U.S. Department of Commerce
U.S. Department of Commerce
 Rezon Bio
Rezon Bio