UK approves Biontech/Pfizer Covid-19 vaccine (copy 1)
Biontech and Pfizer have received emergency approval for their COVID-19 vaccine in the UK. Mass immunization could start next week, the first in Western countries.
Biontech SE in Mainz, Germany, and Pfizer Inc. announced that the Medicines & Healthcare Products Regulatory Agency (MHRA) of the United Kingdom has granted a temporary emergency approval for their mRNA-based COVID-19 vaccine candidate BNT162b2. This is the world’s first emergency approval of a pandemic vaccine in Western countries. The decision of the MHRA is based on the phase III trial results in a rolling review process. The UK regulatory body was given power to approve the vaccine by the government under special regulations before 1 January, when MHRA will become fully responsible for medicines authorisation in the UK after Brexit. According to the companies, the data from the Phase III study had shown a 95 percent immunisation in volunteers without previous SARS-CoV-2 infection. MHRA is currently conducting rolling reviews of vaccine candidates from Moderna and AstraZeneca as well.
"Today’s Emergency Use Authorization in the U.K. marks a historic moment in the fight against COVID-19. This authorization is a goal we have been working toward since we first declared that science will win []," said Albert Bourla, CEO of Pfizer. Ugur Sahin, CEO and co-founder of Biontech, said: "The Emergency Use Authorization in the U.K. will mark the first time citizens outside of the trials will have the opportunity to be immunized against COVID-19."
In July 2020, Pfizer and Biontech signed a supply contract with the UK for 30 million doses of the vaccine. The volume was increased to 40 million doses in October. Deliveries are expected to start within the next few days and to be completed in 2021. The approval now paves the way for mass immunization to start next week for those most at risk.
The European Union had ordered 160 million doses as well. These deliveries could start at the end of December, subject to approval by the European Medicines Agency (EMA). According to the German research ministry, an official public hearing for the Pfizer/Biontech vaccine candidate is scheduled for 11th December and a final decision is expected by 29th December. EMA this week also announced it has received applications for conditional marketing authorization from Moderna and rolling reviews of AstraZeneca and Janssen vaccines are underway. A meeting to review Moderna’s vaccine has been tentatively scheduled for 12 January, EMA’s Executive Director Emeritus Cooke said.
Pfizer and Biontech have also submitted an application for emergency approval to the Food and Drug Administration (FDA) in the USA. In addition, further rolling approval processes are ongoing worldwide.


 eXmoor Pharma
eXmoor Pharma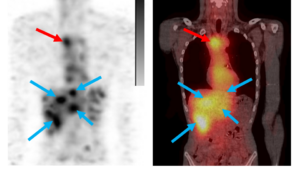 Molecular Partners AG & NuMeRI, presentation Feb. 2026
Molecular Partners AG & NuMeRI, presentation Feb. 2026