Santhera finds Chinese marketing partner for vamorolone
Swiss Santhera Pharmaceuticals has entered into a $124m licence deal with Chinese Sperogenix Therapeuticis to market its DMD drug vamorolone.
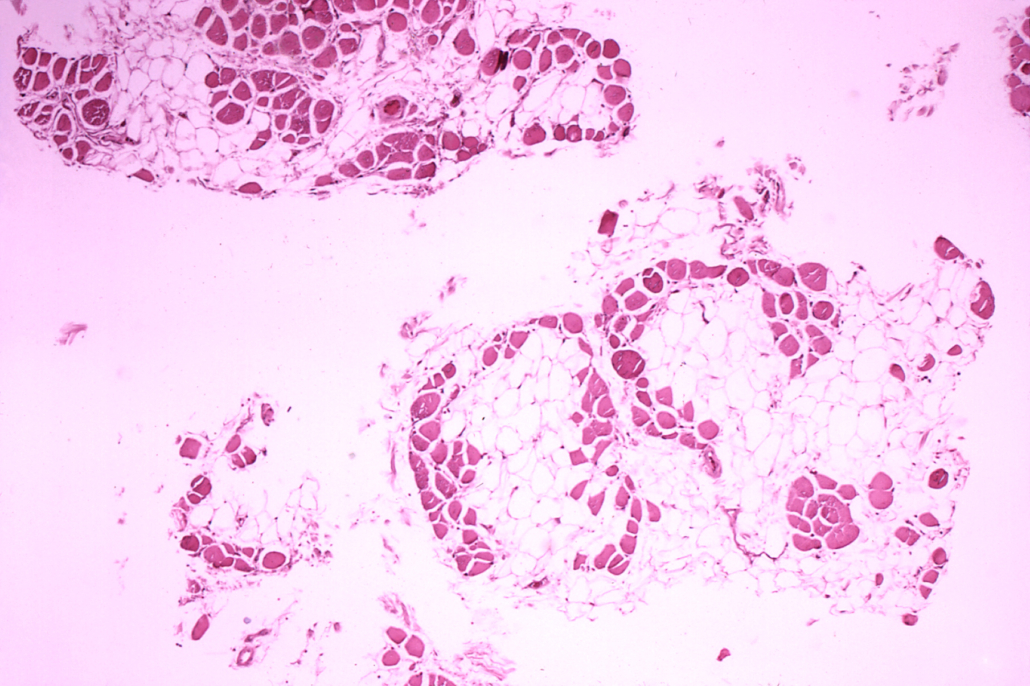
Duchenne muscular dystrophy (DMD) is a rare inherited X-chromosome-linked disease, which almost exclusively affects males. DMD is characterised by inflammation which is present at birth or shortly thereafter. Inflammation leads to fibrosis of muscle and is clinically manifested by progressive muscle degeneration and weaknes
Santhera, which licensed the drug from Idorsia that is intended for around 200 applications against inflammatory diseases, will commence a rolling NDA submission in the US in Q1/2022, paving the way for a first launch as early as beginning of 2023 in the US, followed by a European marketing authorisation application in Q2/2022. Santhera estimates the peak product sales potential for vamorolone in the indication DMD alone to be in excess of US-$500m in the US and the largest five European countries combined.
Vamorolone binds to the same receptor as corticosteroids but modifies its downstream activity and as such is a dissociative agonist. This mechanism has the potential to dissociate’ efficacy from typical steroid safety concerns and therefore vamorolone could emerge as a promising alternative to existing corticosteroids, the current standard of care in children and adolescents with DMD. In the pivotal VISION-DMD study, compared to prednisone, vamorolone showed comparable efficacy, improvements on multiple safety parameters and was associated with fewer adverse events.



 Unsplash+
Unsplash+