Roche gets FDA priority review for atezolizumab
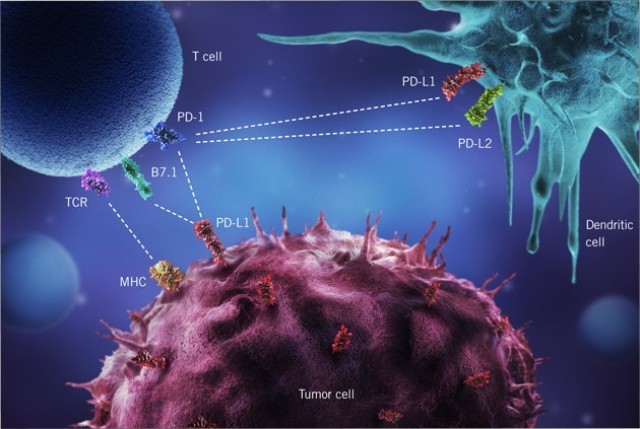
Roche is expanding its indications in cancer immunotherapy. Following FDA approvals of its PD-L1 checkpoint inhibitor atezolizumab in NSCLC and bladder cancer patients refractive to platinum-based chemotherapy, the Swiss pharma major is awaiting a FDA decision on whether the antibody is suitable as first-line treatment in a further subpopulation of bladder cancer patients.
Based on results of a single arm Phase II study, the FDA granted atezolizumab Priority Review in an additional type of advanced bladder cancer. While the agency granted accelerated market approval in May 2016 for the anti-PD-L1 antibody in patients who were treatment-refractive to platinum-based chemotherapy in neoadjuvant or adjuvant setting, the current priority review extends treatment options. Until April 2017, the agency will decide on the approval of atezolizumab in patients with bladder cancer not eligible for cisplatin chemotherapy (first-line treatment) or disease progression after 12 months of cisplatin therapy before or after surgery.
Roche’s supplemental biologics license application (sBLA) submission for atezolizumab is based on results from the Phase II IMvigor210 study. IMvigor210 is an open-label, single-arm Phase II study that evaluated the safety and efficacy of atezolizumab in people with locally advanced or metastatic bladder cancer, regardless of PD-L1 expression. The primary endpoint of the study was objective response rate (ORR). Secondary endpoints included duration of response (DOR), overall survival (OS), progression-free survival (PFS) and safety.



 Unsplash+
Unsplash+