Roche’s NSLC mAb outperforms chemo
Roche is making up lost ground in immuno-oncology. The Swiss company's PD-L1 directed immunotherapy atezolizumab is more effective in combatting lung cancer than standard chemotherapy.
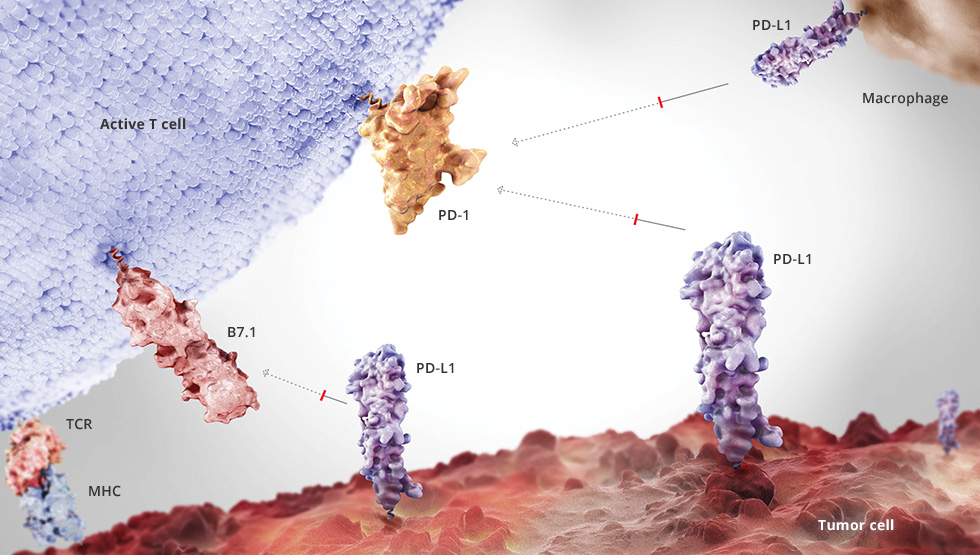
For almost three years, cancer market leader Roche has lagged behind Bristol Myers Squibb in cancer immunotherapy. Now the Swiss are back. While BMS’s anti-PD1 antibody nivolumab failed this August in a Phase III trial assessing its effectiveness as a monotherapy in 850 patients with previously untreated advanced non-small cell lung cancer, Roche’s anti-PDL1-antibody atezolizumab showed significant survival advantage compared to chemotherapy regardless of PD-L1 status.
According to results from a primary analysis of the OAK study, atezolizumab improved overall survival by 4.2 months compared to docetaxel chemotherapy (OS: 13.8 vs 9.6months; HR=0.73, 95% CI: 0.62-0.87) regardless of their levels of programmed death-ligand 1 (PD-L1) expression. However, the OAK study evaluated people with NSCLC whose disease had progressed on or after treatment with one or more platinum-based chemotherapy in squamous and non-squamous disease (2nd or 3rd line therapy) while BMS tested nivolumab as first-line therapy. Results were presented at the European Society of Medical Oncology (ESMO) 2016 Annual Meeting in Copenhagen, Denmark.
The FDA granted Breakthrough Therapy Designation (BTD) for atezolizumab for the treatment of people with PD-L1 positive non-small cell lung cancer (NSCLC) whose disease has progressed during or after platinum-based. Roche’s Biologics Licence Application (BLA) for NSCLC was granted Priority Review with an action date of 19 October 2016.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink