PCR-test reveals risk of genotoxity of genome editing
German researchers have presented a new method to assess on-target and off-target effects of CRISPR-Cas nucleases or TALENs.
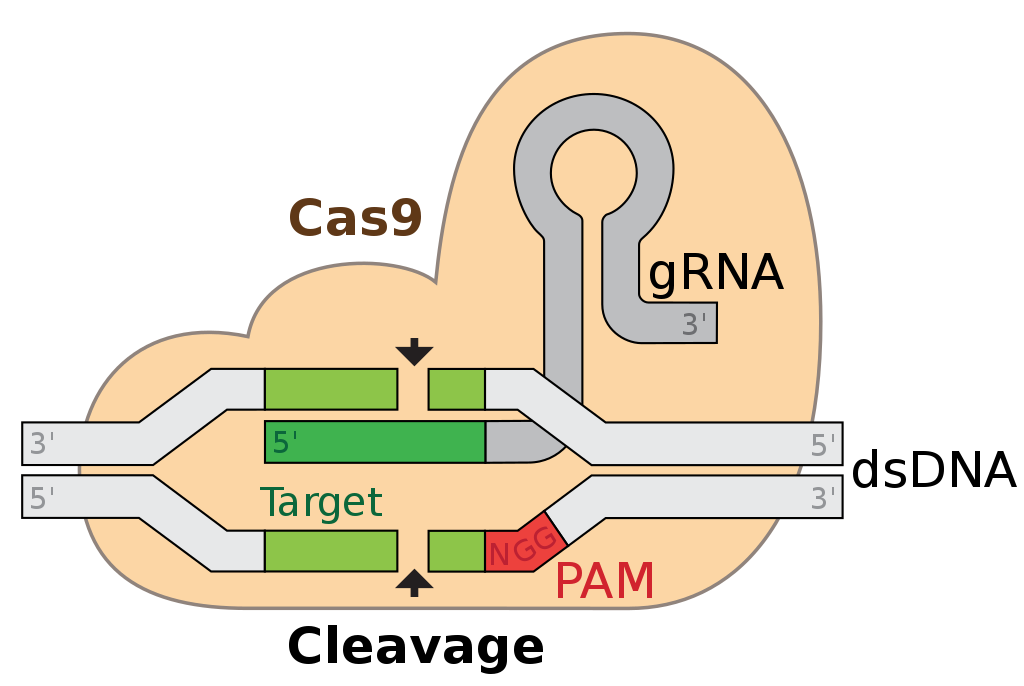
The new method called single targeted linker-mediated PCR sequencing (CAST-Seq), developed at University Hospital Freiburg, Germany, is set to become an important new tool to evaluate the safety of gene and cell therapies, which are used for the treatment of hereditary diseases and cancers. Whether and how precisely gene scissors such as CRISPR-Cas9 used in these treatments cut exclusively at the desired location in the genome has so far been insufficiently investigated. CAST-Seq for the first time can identify the location and extent of faulty cuts and potentially dangerous rearrangements in the genome.
"The CAST-Seq method allows it to assess the risk of off-target effects before it is used clinically in patients," explaied Prof. Dr. Toni Cathomen, Director of the Institute for Transfusion and Gene Therapy at the University Medical Center Freiburg. Using the method to assess CRISPR/Cas and TALEN-mediated genomic rearrangements in blood stem cells, CAST-Seq detected translocations in 0%-0.5% of gene-edited human CD34+ HSCs, and up to 20% of on-target loci harbored gross rearrangements. Moreover, CAST-Seq detected distinct types of chromosomal aberrations, such as homology-mediated translocations, that are mediated by homologous recombination and not off-target activity.


 eXmoor Pharma
eXmoor Pharma Molecular Partners AG & NuMeRI, presentation Feb. 2026
Molecular Partners AG & NuMeRI, presentation Feb. 2026