Ose Pharma gets clinical milestone for IL7R antagonist
Ose Immunotherapeutics has got the first milestone payment within its €272m deal with Servier inked in 2016.
Under the deal inked in 2016, immuno-oncology and autoimmune specialist Ose Immunotherapeutics develops its IL-7-receptor antagonist OSE-127 until Phase II proof of concept in ulcerative colitis while Servier has the option to licence it for global commercialisation in autoimmune disorders and chronic inflammation. The Nantes-based company, who enroled the first volunteers into a Phase I trail in December 2018, now achieved step one – an undisclosed development milestone – of the two-step option agreement cashing in a €10m milestone payment.
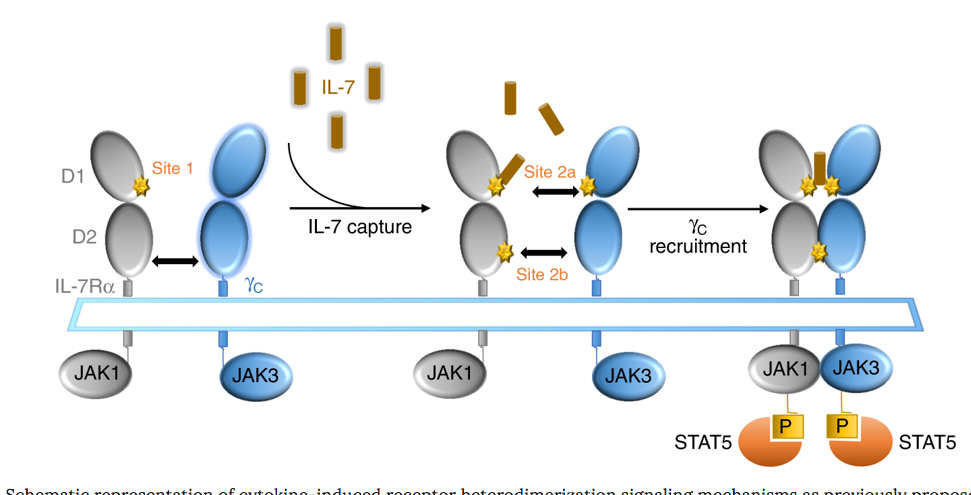
OSE-127 is an anti-CD127 monoclonal antibody targeting the alpha chain of the interleukin-7 receptor (IL-7R) on T effector cells. Binding of OSE-127 to CD127 inhibits receptor dimer internalisation and was recently shown to prevent the migration of pathogenic T lymphocytes while preserving regulatory T lymphocytes, which carry only few IL.7 receptors at its surface. Experiments in non-human primates suggest that blocking of receptor signalling shifts the Teff/Treg balance towards the immune-dampening Tregs, which could have a positive impact in a range of autoimmune diseases including autoimmune colitis, multiple sclerosis, type-1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, and primary Sj?gren’s syndrome.
Servier also wants to develop OSE-127 in Sjögren’s syndrome, the second most frequent auto-immune disease.



 Unsplash+
Unsplash+