Obesity gene therapy enters Phase I testing
After securing an equity investment from French pharma giant Sanofi, Italian gene therapy company Resalis Therapeutics srl has begun enrolling participants for a Phase I study of its obesity gene therapy, RES-010, which converts white adipocytes into brown adipocytes.
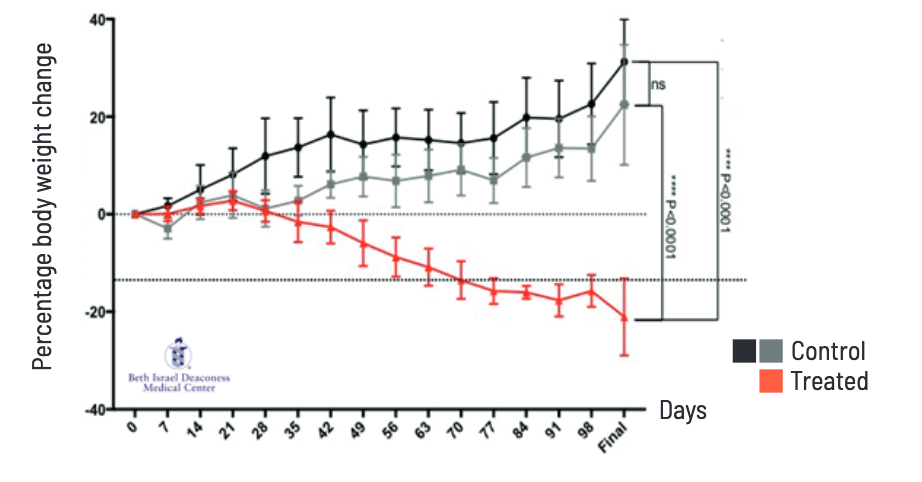
Resalis Therapeutics srl has initiated a first-in-man Phase 1 trial to test whether there is a therapeutic window for its obesity gene therapy RES-010, a non-coding RNA-based compound that targets the miRNA-22 signalling pathway designed to provide a disease-modifying approach to obesity treatment. In preclinical studies, the start-up has demonstrated that RES-010 reduces fat mass, preserves lean body mass, and enhances energy expenditure. By targeting fat reduction across various parts of the body, including visceral and hepatic stores, RES-010 might potentially complement existing GLP-1 receptor-agonist-based therapies, and support sustainable, long-term weight management.
The Phase 1 trial (EUCT No: 2024-514871-17-00) is designed to explore the safety, tolerability, pharmacokinetics, pharmacodynamics, and first hints to efficacy of RES-010 in healthy, overweight, and moderately obese volunteers. The Phase 1 trial is a randomised, double-blind, placebo-controlled study conducted in the Netherlands. It consists of two parts that aim to find a therapeutically safe and efficient dose of RES-010: a single ascending dose (SAD) phase and a multipleascending dose (MAD) phase. In the SAD phase, up to 48 healthy male and female participants will receive ascending single doses of RES-010 to determine the maximum dose and pharmacokinetics. The subsequent MAD phase will involve 24 overweight and 8 moderately obese participants who will receive multiple doses to further assess RES-010’s safety and tolerability. The trial’s primary objective is to assess the safety and tolerability of RES-010, while also evaluating its pharmacokinetic profile. Additionally, exploratory endpoints include assessing the effect of RES-010 on specific metabolic markers, change in lipid metabolism, body weight, appetite, and glucose tolerance.
Targeting the miR-22 pathway adds a treatment option because – in contrast to existing therapies – it selectively reduces fat while preserving muscle mass, according to Almut Nitsche, Chief Medical and Development Officer of Resalis Therapeutics. “With RES-010, we expect to shift the focus from managing symptoms in the short termto achieving durable, long-term impact on obesity,” said Alessandro Toniolo, Chief Executive Officer of the company that recently got a financing by French drug giant Sanofi to continue testing upon Phase II proof of concept. miR-22 ist a master regulator of lipid biosynthesis, mitochondrial function, and adipose tissue transformation.
Data provided by the combined SAD/MAD study are expected by mid-2026.


 SANOFI
SANOFI IQVIA
IQVIA White House
White House