Novel strategy targets chronic liver diseases
A resesarch team headed by HepaRegeneriX Board Member Lars Zender has presented a new approach to treat hepatocellular carcinoma (HCC) by disturbing the lipid metabolism of tumour cells.
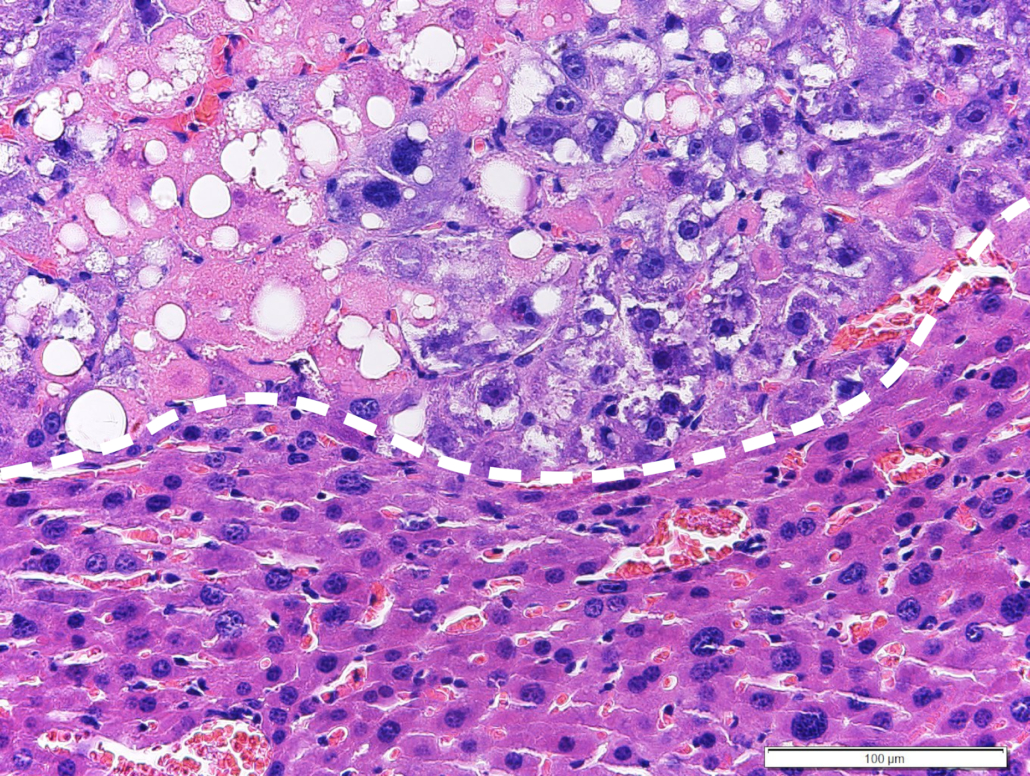
The approach published in Nature Cancer leads to a cell-toxic accumulation of saturated fatty acids that drives tumour cells into apoptosis. HCC accounts for 85-90 percent of all cases of primary liver cancers.
Specifically, Zender and colleagues activated LXRalpha, a driver of fatty acid synthesis. As cells tend to escape accumulation of toxic amounts of saturated fatty acids by conversion into unsaturated fatty acids using the enzyme stearoyl-CoA desaturase-1 (SCD1), however, a second step was necessary to induce apoptosis: inhibition of the SCD-1 activator Raf-1 kinase.
Combination of LXR agonists and Raf inhibitors showed strong efficacy against liver cancer in steatohepatitis-induced HCC mouse models and xenograft models of human HCC while being well tolerated and capable of overcoming therapy resistance in HCC, which was caused by non alcoholic steatohepatitis (NASH), a medically underserved condition.
Meanwhile, UK researchers have for the first time proved that organoids grown from bile duct epithelial cells, so called cholangiocytes, can be used for the ex vivo repair of damaged bile ducts in transplanted human livers. Provided in vivo engraftment, survival and function of organoids in humans can be established in the future, the new technology would ultimately increase the number of useable organs on the transplant waiting list.
Using single-cell RNA sequencing and a mouse model of biliary injury, Fotios Sampaziotis and colleagues found that organoids grown from primary human cholangiocytes retain their plasticity, allowing cells from one region to repair different regions of the biliary tree. The findings provide proof of concept that cholangiocytes from non-diseased areas, such as the gallbladder, could be used in cell-based therapies for cholangiopathies affecting bile ducts within the liver.



 Unsplash+
Unsplash+