Medicxi put €40m into two Sosei spin off companies
Sosei Group Corporation has spun out two programmes of its neurology pipeline into two newly created companies funded by venture fund Medicxi.
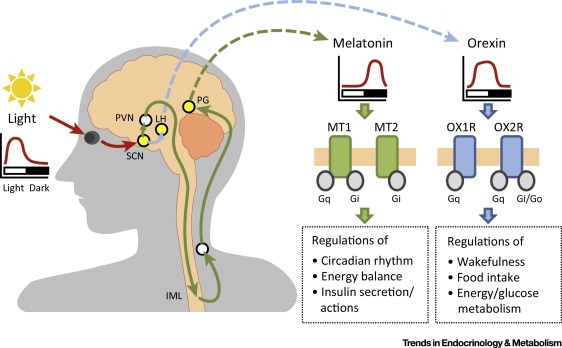
The newly created companies Orexia Ltd and Inexia Ltd aim to develop orally or intranasally administered drug candidates to treat the rare sleep disorder narcolepsy using Sosei Heptares‘ orexin agonist platform, which targets the G protein-coupled receptors Orexin OX1 and OX2 (HCTR1 and HCTR2) in the hypthalamus. Medicxi agreed to invest up to €40m in both companies. Mario Alberto Accardi, who helped structure the deal on behalf of Medicxi, will become CEO of both companies.
Under the terms of the agreement, Orexia and Inexia will gain a portfolio of related patents from Heptares Therapeutics Ltd and have the right to exploit a series of Orexin OX1 and OX2 positive modulators and products derived therefrom, including dual OX1/OX2 agonists of Heptares, as well as access to proprietary know-how and development capabilities. While Orexia will focus on the development of oral therapies able to cross the blood-brain barrier (BBB), Inexia will focus on the development of candidates for intranasal delivery using the Optinose Exhalation Delivery System (EDS). Heptares Therapeutics will retain an equity holding in both companies and will receive R&D payments as well as milestone payments.
Orexia and Inexia will use the proceeds from Medicxi for lead optimisation, formulation and clinical development towards Phase IIb POC. According to SoseiHeptares narcolepsy is the primary target indication. Narcolepsy is a socially debilitating disorder characterised by an inability to properly maintain wakefulness, and a pathological intrusion of signs of REM sleep into wakefulness. Narcolepsy is a non-progressive, life-long condition. A vast majority (>90%) of human narcoleptics lacks detectable levels of the orexin peptides in the cerebrospinal fluid due to a highly specific degeneration of orexin neurons, indicating that human narcolepsy is an orexin deficiency syndrome. Most patients with narcolepsy have diminished levels of orexin A concentrations in cerebral spinal fluid, which binds to both OX1 and OX2 in the hypothalamus.
Thus it is probable that the drug cancidates in question represent orexin A mimetics. OX agonists enhance wakefulness, reduce jet leg or anestesia recovery time. Furthermore OX2 agonist can help reduce fat accumulation. According to estimates, narcolepsy affects about 236,000 patients in the US and the EU, is heavily underdiagnosed – durgs to treat the disorder are forcasted to achieve a market value of €2.5bn by 2022.
Orexia and Inexia will run out-sourced drug development programmes, leveraging an experienced team of drug developers within Sosei Heptares and additional contributions from a group of leading experts in the orexin space. Both companies follow Medicxi’s asset-centric virtual model utilising minimal infrastructure and thus increasing capital efficiency.
Currently, there are some interesting therapy options of narcolepsy that could replace amphetamine ("speed")-based treatment:
Pitolisant is an EU approved (2016) histamine H3 receptor inverse agonist that activates histamine neurons, under development in the US to treat excessive daytime sleepiness and cataplexy in people with narcolepsy.
Solriamfetol (JZP-110) is a wake-promoting agent, a dopamine and nonrepinephrine reuptake inhibitor to treat excessive daytime sleepiness in people with narcolepsy and obstructive sleep apnea. Jazz Pharmaceuticals filed for and NDA at the FDA last year.
Sodium oxybate is currently tested in Phase III clinical trails for the treatment of excessive daytime sleepiness (EDS) and cataplexy
Furthermore, two OX2 agonists, YNT-185 and TAK-925 have been shown to reduce daytime sleepiness in mice models for narcolepsy.


![DBT26_Snap_1600[45] DBT26_Snap_1600[45]](https://european-biotechnology.com/wp-content/uploads/2026/03/DBT26_Snap_160045-300x188.jpg)
 Unsplash+
Unsplash+ Marcus Krauss - stock.adobe.com
Marcus Krauss - stock.adobe.com