
Lilly pays US$415m for rights to ATLX-1282
Building on the January 2025 ALS drug discovery collaboration with Alchemab Therapeutics, Eli Lilly & Company has secured rights to the first IND-ready program under a US$415m licensing agreement.
Cambridge-based Alchemab Therapeutics Ltd has entered into a licensing agreement with Eli Lilly and Company for ATLX-1282, Alchemab’s first-in-class IND-ready programme for amyotrophic lateral sclerosis (ALS) and other neurodegenerative conditions. Alchemab’s platform uses the power of human immune evolution to identify and develop naturally occurring therapeutic antibodies from resilient individuals.
The transaction is worth up to a total of US$415m, including an undisclosed upfront payment, potential discovery, development, and commercialisation payments and royalties. Under the terms of the agreement, Alchemab will be taking the programme through the risky first-in-man Phase I safety and dose-finding studies after which Lilly will lead all further development and commercialisation.
Alchemab’s platform uses machine learning to analyse the human immune response and identify antibodies that are uniquely associated with resilience to untreatable diseases. The company has 6,000 carefully selected and highly curated patient samples across neurodegeneration, immunology, oncology and healthy aging in its portfolio. Weaving together lab-based protein science and biology with machine learning, human samples and proprietary data analysis, and leveraging Nvidia’s supercomputer in Cambridge, Alchemab has sequenced and analysed millions of antibody sequences to unveil novel targets and antibodies with unique mechanisms of action.
Through its research, Alchemab has identified an antibody in people with mutations that normally lead to frontotemporal dementia (FTD), but who remain well into old age. These samples were sourced from a collaboration with the Genetic Frontotemporal Initiative (GENFI) consortium, which has built the largest global cohort of FTD patients.
Starting from the antibody sequence, Alchemab was able to identify the target and has subsequently demonstrated its importance in neuroprotection, and across multiple neurodegenerative conditions including ALS and FTD.
Alchemab’s Chief Executive Officer, Jane Osbourn, commented: “As the first programme from our highly novel platform, this is a landmark transaction for Alchemab. With Lilly’s deep expertise in neurological conditions, they are ideally placed to speedily advance ATLX-1282 through the clinic, and maximise the potential to help patients.


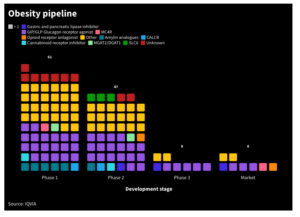 IQVIA
IQVIA Momoneymoproblemz - wikimedia
Momoneymoproblemz - wikimedia stock.adobe.photo.com/Popelniushka
stock.adobe.photo.com/Popelniushka