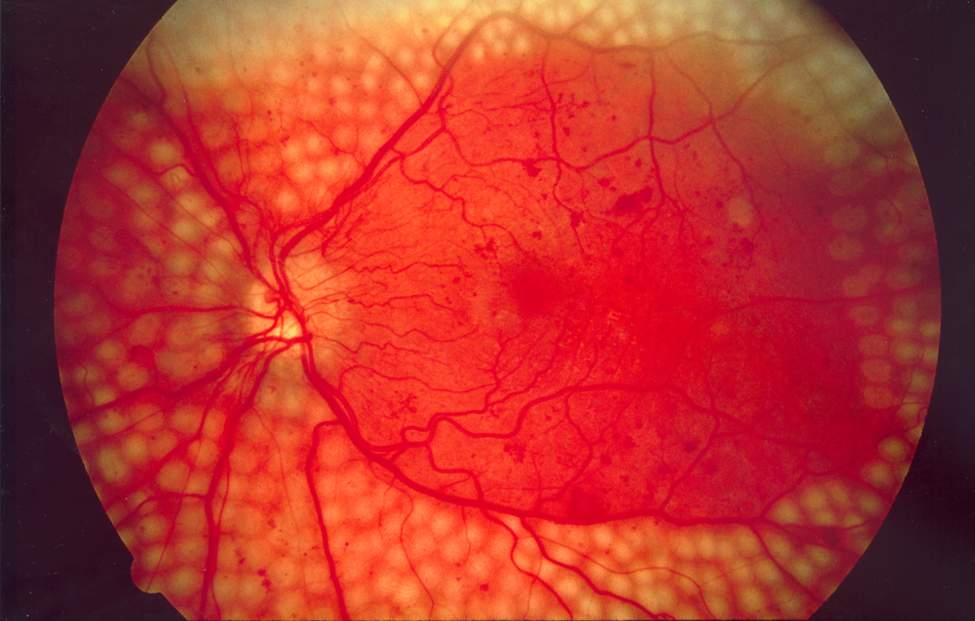
Eylea met Phase III endpoint in diabetic retinopathy
VEGF blocker Eylea (aflibercept), which is co-developed for eye diseases by Bayer HealthCare and Regeneron under a deal signed in 2006, has met the 24-week primary endpoint of the pivotal Panorama Phase III trial.
According to Regeneron, the fusion protein of extracellular VEGF-receptor 1 and 2-fragments with the FC portion of IgG1, improved the Diabetic Retinopathy Severity Scale (DRSS) in 58% of patients with non-proliferative diabetic retinopathy without diabetic macular edema vs placebo (6%) after 24 weeks of treatment with 4-5 injections on average. After the second readout at 52 weeks of treatment this year, Regeneron plans to apply for label extension of Eylea to the FDA.
In the US, the VEGF blocker is approved as a treatment for wet age-related macular degeneration and diabetic macular edema in patients with diabetic retinopathy or retinal vein occlusion. Bayer has the ex-US commercialisation rights to the VEGF blocker.
However, as VEGF inhibitors in eye diseases are challenged by off-label use of Roche’s cancer drug Avastin, which has been prooved to be as effective, but at least 20 times cheaper than approved treatments such Eylea or Lucentis (ranibizumab, Roche/Genentech), companies seek to find alternative treatments the outperform such off-label competitors.
In February, Roche presented Phase II results of a bispecific VEGF/Ang1 blocker code-named RG7716 that outcompeted ranibizumab in diabetic macular. The Crossmab format developed at Roche’s rRED division has passed Roches internal lifecycle investment point (LIP) and will be pushed through Phase III developments. In people with vision loss from diabetic macular edema (DME), monthly injections with intravitreal RG7716 resulted in significant improvements in visual acuity gains compared with ranibizumab alone. At 24 weeks a 6mg dose of RG7716 improved Best Corrected Visual Acuity (BCVA) by 13.9 chart letters vs 10.3 letters in patients treated with 0.3mg ranibizumab.
A patch of human embryonic stem cell (hESC)-derived retinal pigment epithelial (RPE) cells, however, improved visual acuity in two patients with severe wet age-related macular degeneration in a Phase I trial. Albeit development of PF-05206388 has been terminated by Pfizer due to strategic commercial reasons and rights have been transfered back to its originators at University College London, the patch improved best corrected visual acuity (BCVA) at 12 months by 29 letters in the first patient and by 21 letters in the second patient. The patch also improved the patients’ reading speed to 82.8 and 47.8 words per minute, respectively, from baseline reading speeds of 1.7 and 0 words per minute, the researchers, who will restart development soon, reported in Nature Biotechnology.


 Unsplash+
Unsplash+
