€7.34m for cancer vaccine developer
Having secured a €2.785m grant from the EU and €4.55m from a Series B round, MaxiVAX is going full steam ahead with its Phase II cancer vaccine programmes.
Private clinical-stage biotech MaxiVAX announced that it has been awarded a European Commission grant of €2,785,000, thanks to the Horizon 2020 EIC Accelerator Programme. In addition, the company successfully closed a Series B2 round of CHF5m (€4.55m). Founded by the father and son team Bernard and Nicholas Mach, the Geneva-based biotech is focused on developing personalized anti-cancer vaccination.
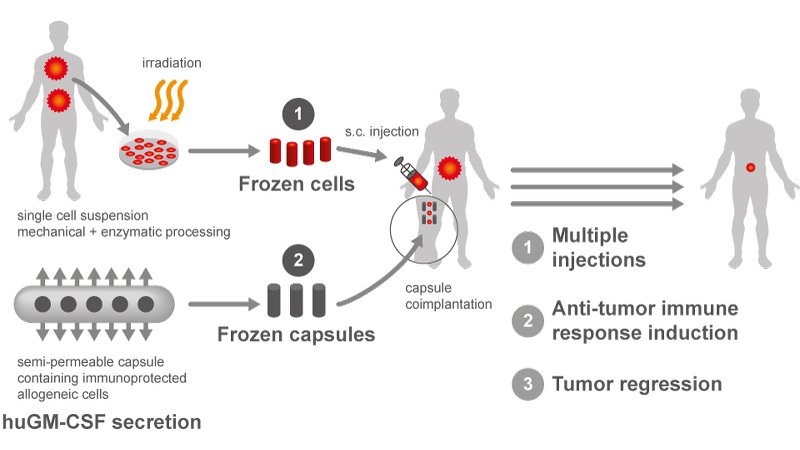
MaxiVAX will use the funds to support the clinical development programmes which include the multi-centric efficacy Phase II trial for advanced, refractory head and neck cancers that is currently ongoing in Switzerland. The company will also start preparations for an international Phase II clinical study in a yet to be disclosed rare cancer indication, for which the FDA accepted the Investigational New Drug application in July 2018. The new funds will also be used to scale up production of the company’s lead compound, MVX-ONCO-1. MVX-ONCO-1 is the first personalised cell-based cancer immunotherapy using encapsulation cell technology. Therapy is individualised and can be applied to any cancer type.
This important and welcome funding now enables us to move forward and accelerate our clinical programmes in Switzerland, Europe and the US, commented MaxiVAX CEo Dimitrios Goundis. We are encouraged by the progress in our ongoing Head & Neck Phase 2 trial, and look forward to its completion in 2021.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink