EU orders up Valneva’s inactivated Covid-19 vaccine
The EU has secured 60 million doses of Valneva's potential Covid-19 vaccine VLA2001. When approvied, it would be the first inactivated vaccine on the EU market.
The European Commission has approved an agreement with Valneva under which the French vaccine maker would supply up to 60 million doses of VLA2001, its inactivated COVID-19 vaccine candidate. According to the contract, EU member states could purchase almost 27 million doses in 2022. It also includes the possibility to adapt the vaccine to new variant strains, and for EU countries to make a further order of up to 33 million additional vaccine doses in 2023.
VLA2001 is currently the only whole virus, inactivated vaccine candidate against COVID-19 in clinical trials in Europe. Using inactivated viruses is a traditional vaccine technology that is used for most of the flu vaccines as well as many childhood vaccines. We continue to receive messages from people across the world who are waiting for an inactivated vaccine, stressed Thomas Lingelbach, Valneva’s CEO. Our Phase 3 results confirmed the advantages often associated with inactivated vaccines and we continue to believe that our differentiated vaccine candidate could make an important contribution to the global fight against the COVID-19 pandemic. The company is preparing to commence rolling submission for conditional approval with the European Medicines Agency. Valneva reported positive Phase 3 results for VLA2001 in October 2021.
The EU’s contract with Valneva comes in addition to an already secured broad portfolio of vaccines to be produced in Europe, including the contracts already signed with AstraZeneca, Sanofi-GSK, Janssen Pharmaceutica, BioNtech-Pfizer, CureVac, Moderna, and Novavax. Our broad portfolio will help us to fight COVID and its variants in Europe and beyond, said Ursula von der Leyen, President of the European Commission. The pandemic is not over. Everyone who can should get vaccinated.


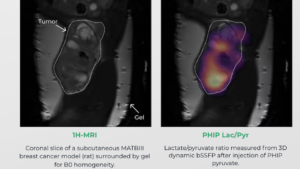 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics