EMA starts rolling review for Sputnik V vaccine
EMA's human medicines committee has started a rolling review of Sputnik V, a COVID-19 vaccine developed by Russia's Gamaleya National Centre of Epidemiology and Microbiology.
The EU applicant for Gam-COVID-Vac is R-Pharm Germany GmbH. The CHMP‘s decision to start the rolling review is based on results from laboratory studies and clinical studies in adults. These studies indicate that Sputnik V triggers the production of antibodies and immune cells that target the SARS-CoV-2 coronavirus and may help protect against COVID-19.
According to published Phase III data, the vaccine showed an efficacy of nearly 92%. Sputnik V is made up of attenuated adenoviral vectors, Ad26 and Ad5. These adenoviruses have been modified to contain the gene for making the SARS-CoV-2 spike protein: Ad26 is used in the first dose and Ad5 is used in the second to boost the vaccine’s effect.
Once it has been given, the vaccine delivers the SARS-CoV-2 gene into cells in the body. The cells will use the gene to produce the spike protein. The person’s immune system will treat this spike protein as foreign and produce natural defences ? antibodies and T cells ? against this protein.
EMA will evaluate data as they become available to decide if the benefits outweigh the risks. The rolling review will continue until enough evidence is available for formal marketing authorisation application.
As with other vaccines, there have been attempts to exert political influence on the approval decision, which is based on standardized scientific criteria. Emergency mass-distribution of the vaccine began in December 2020 in multiple countries including Russia, Argentina, Belarus, Hungary, Serbia and the United Arab Emirates. As of March 2021, thirty nine countries have granted Sputnik V emergency use authorization while over a billion doses of the vaccine were ordered for immediate distribution globally.


 eXmoor Pharma
eXmoor Pharma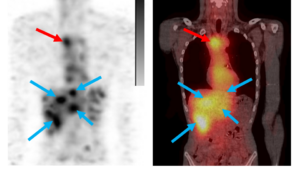 Molecular Partners AG & NuMeRI, presentation Feb. 2026
Molecular Partners AG & NuMeRI, presentation Feb. 2026