Cytovation ASA bags additional US$6m to drive Phase II study
Cytovation ASA has baged US$6m to advance Phase II development of its bi-specific pore-forming peptide candidate CY-101 in patients with adrenocortical carcinoma.
The additional US$6m (NOK62m) for the Norwegian cancer specialist Cytovation ASA were largely provided by Series A-financing lead investor Sandwater and other existing investors such as the Norwegian Cancer Society and brings Cytovation total capital to US$34m. It forms the basis for a multi-centre Phase II trail conducted by Cancer Research UK with the peptidic beta-catenin blocking cancer vaccine CY-101 in patients with adrenocortical carcinoma. The study is due to start in late 2025 and the partners expect. to present first clinical readouts next year.
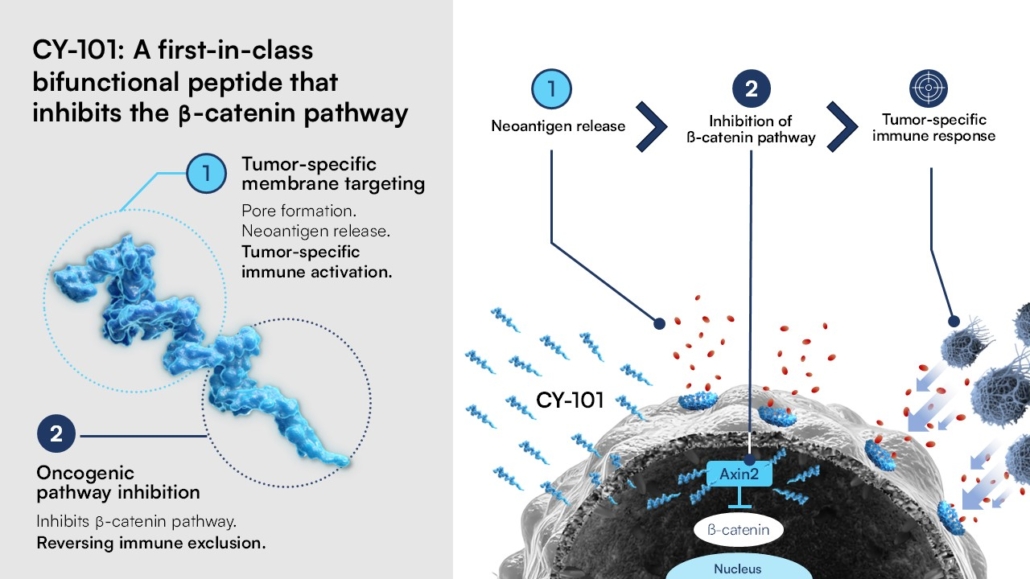
Cytovation will present new preclinical data on CY-101 in adrenocortical carcinoma and other cancer types driven by a dysfunctional Wnt/β-catenin pathway, including colorectal cancer, at AACR annual meeting in Chicago. CY-101 is a membranolytic inhibitor, that is it forms pores in cancer cells leading to the release of cancer neoantigens into the tumour microenvironment that trigger an immune response. In addition, CY-101 inhibits the oncogenic Wnt/beta-catenin signaling pathway, which is frequently dysregulated in beta-catenin-driven cancers such as colorectal carcinoma (CRC), adrenocortical carcinoma (ACC), and melanoma. Given the high prevalence of beta-catenin mutations in these tumours, targeting this pathway offers significant therapeutic potential, according to Lars Prestegarden, CEO of Cytovation.
That data to be presented at AACR at the end of April demonstrate that CY-101 induces a dose-dependent cell death across mouse and human beta-catenin-driven CRC, ACC, and melanoma cell lines in vitro following suppression of the Wnt/beta-catenin pathway. In preclinical animal models, CY-101 elicited a potent anti-tumour activity by completely eradicating ACC tumors and significantly enhancing the efficacy of anti-PD-1 therapy in CRC and melanoma models. Notably, CY-101 treatment reshaped the tumour immune landscape by modulating cytokine expression, leading to increased infiltration of cytotoxic CD8-expressing T cells. Mechanistically, CY-101-treated tumours exhibited downregulation of Wnt/beta-catenin downstream target genes. These new preclinical results support Cyrovation’s observations seen in the first in human CICILIA Phase I/IIa trial, which reported clinical activity in ACC and melanoma patients.
According to Cytovation, the new findings support the potential of CY-101 as a novel therapeutic for difficult-to-treat cancers such as ACC, and more broadly for tumours with dysregulated Wnt/β-catenin signaling, like CRC and hepatocellular carcinoma (HCC), among others.




