
Confo Therapeutics and Lilly partner to advance CFTX-1554
Drug developer Confo Therapeutics and Eli Lilly & Company have inked a worldwide licensing agreement to co develop Confo's neurophatic pain candidate CFTX-1554.
Under the agreement, Confo Therapeutics BV got a $40m upfront and milestone payments totalling $590m for licencing its clinical stage angiotensin II type 2 receptor (AT2R) blocker CFTX-1554 and backup candidates with an option to get further $590m in case Lilly licences a second candidate from the non-opoid program against neuropathic pain. According to the contract, Lilly will continue clinical testing beyond the current Phase I study. Additionally, the pharma company will eveluate therapeutic antibody candidates that home in on the same target as CFTX-1554. Lillys Belgian partner Confo maintains a co-investment option to participate in the funding of future development programs after clinical proof-of-concept for additional royalties.
After previous developed therapeutics failed market approval, the new non-opioid approach CFTX-1554 is Confos first product candidate in a Phase 1 first-in-human clinical study. CFTX-1554 should avoid serious side effects of opoids to treat neuropathic pain, like addiction and sedation. The compound binds better than predecessors to its target binding site at AT2R. Confo Therapeutics’ technology stabilises functional conformations of G-protein coupled receptors, thereby enabling the discovery of chemical or biological ligands that are conformationally selective.
Cedric Ververken, CEO of Confo Therapeutics commented: CFTX-1554’s progression through the clinica will benefit from Lilly´s experience and global organization while we will continue to develop and expand our growing, innovative pipeline of GPCR-targeted assets, both small molecules and biologics.


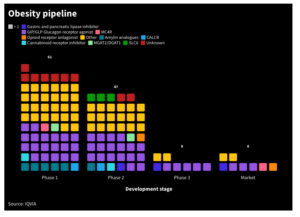 IQVIA
IQVIA Momoneymoproblemz - wikimedia
Momoneymoproblemz - wikimedia stock.adobe.photo.com/Popelniushka
stock.adobe.photo.com/Popelniushka