Cellestia: €20m to get to Phase II
Basel-based biopharma company Cellestia Biotech AG has successfully closed a Series B financing round, raising a total of CHF 20 million.
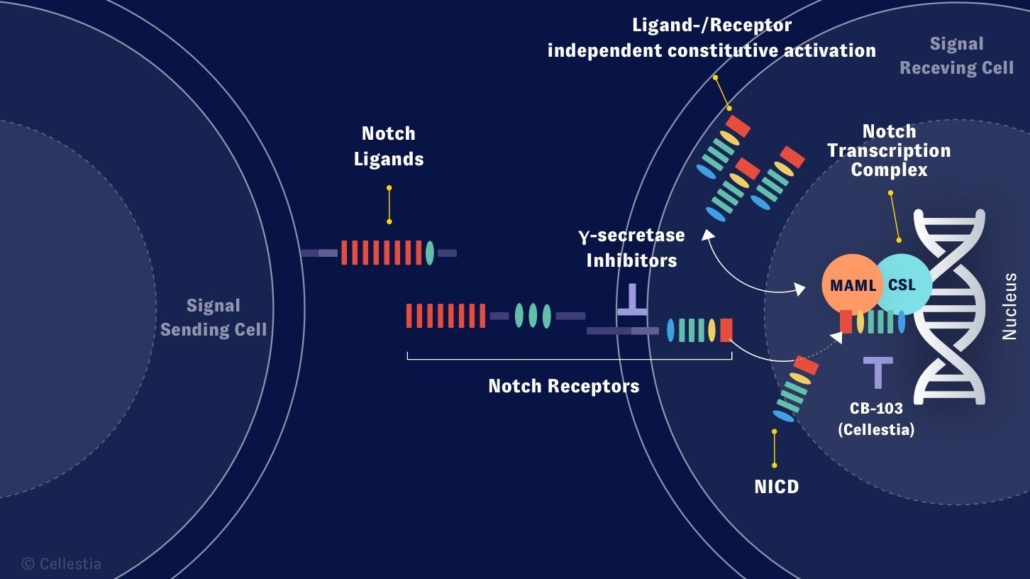
Funds will be used to finance the operations and to advance the ongoing Phase l clinical trial for the company’s programme CB-103 into Phase II. CB-103 is highly selective protein-protein interaction inhibitor targeting an oncogene transcription factor for the precision medicine treatment of specific cancers. The first-in class pan-NOTCH inhibitor is indicated for treatment of patients with NOTCH-driven leukaemias, lymphomas and solid tumours.
The financing round, which has brought the total capital raised by Cellestia to CHF49m (US$50m), was led by FC Capital, PPF Group and Partners Investment. This financing strengthens our balance sheet as we work to achieve important milestones for our clinical candidate CB-103 as well as the follow-up compounds, said Gaudenz von Capeller, CFO of Cellestia Biotech AG.
There is currently no NOTCH-specific treatment available on the market. With more than 250,000 patients diagnosed with NOTCH-driven cancers every year, the market potential for CB-103’s targeted therapy is substantial. Cellestia expects the commercial potential to reach billions in sales annually.


 Unsplash+
Unsplash+
