Arctic Therapeutics ASA kicks off with €26.5m Series A round
Iceland's Arctic Therapeutics has secured €26.5m in a Series A financing aimed to push its oral drug candidates AT-O01 and AT-004 into clinical trails in patients with rare amyloid aggregation diseases including Alzheimer's disease.
The €26.5m Series A round led by an international investor consortium will be used to accelerate the company’s anti-amyloid treatments AT-001 and AT-004 for rare and common forms of dementia. Specifically, EIC Fund, Iceland’s largest privately-held investment firm Kaldbakur, Investcorp-backed Sanos Group, Swiss Cerebrum DAO, The Lurie Family Foundation as well as seed investors and co-founders of Icelandic unicorn, Kerecis, and Copenhagen-listed Chemometec participated in the financing.
The proceeds from the Series A financing will be used to speed up clinical development of Arctic Therapeutic’s two frontrunners, AT-001 and AT-004.
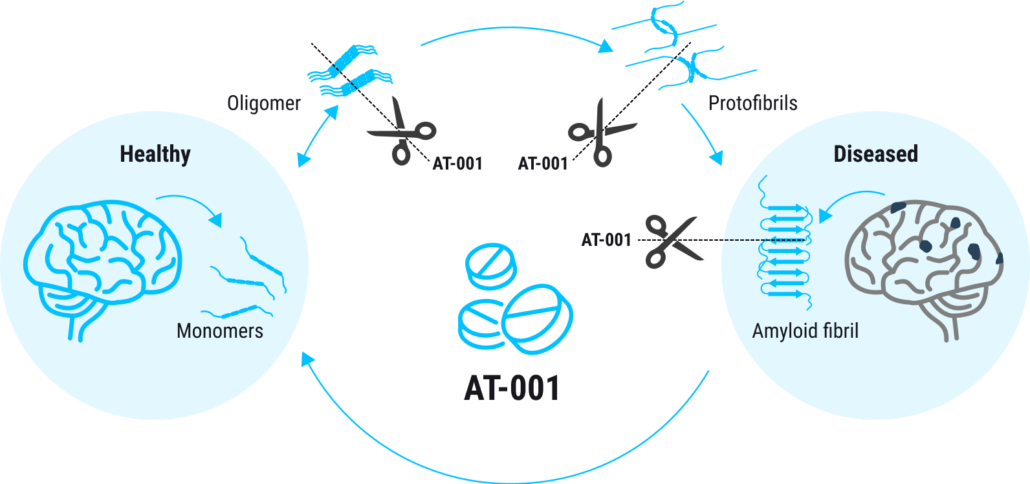
Last year, the European Medicines Agency (EMA) approved a pivotal Phase IIb/III clinical trial evaluating AT-001 for the treatment of Hereditary Cystatin C Amyloid Angiopathy (HCCAA), a rare form of familial dementia. AT-001 is an oral treatment with, so far, no observed side effects. It is designed to address rare and common forms of dementia caused by amyloid-induced angiopathy. Its mechanism of action is focused on the disruption and dissolution of protein oligomers within the brain by reducing disulfide bonds that stabilise protein clumps. The new proceeds allow the start-up to explore the potential of AT-001, which preclincially prevented the aggregation of harmful amyloid proteins in the brain, in other forms of familial dementia and Alzheimer’s disease.
Furthermore, Arctic Therapeutics is also planning to launch a Phase IIa dose escalation trial for AT-004 to demonstrate safety and and provide first hints to efficacy in acne vulgaris before expanding into other inflammatory skin diseases, including atopic dermatitis, rosacea and psoriasis. AT-004 is a topical solution engineered to improve the treatment of multiple skin disorders by leveraging an acetylcholinesterase inhibitory mechanism.
The company has two other clinical programmes dubbed AT-03 (Phase II) to treat autoimmune diseases (MS, RA, SLE) and Parkinson’s, AT-002, designed to treat uveitis and rare auto- inflammatory diseases, is in Phase I developement.
The company uses genetic sequencing to analyse and map the genetic causes of diseases at a granular level using Iceland’s genealogy database, biobank licensed by the Icelandic Ministry of Health and partners with national hospitals, granting fully encrypted access to Iceland’s Electronic Health Records (EHR) database.
Upon the closure of the financing, appointed Jeppe Ragnar Andersen, CEO of Sanos Group, to its board of directors. “Sanos Group’s proven expertise in dermatology and recent acquisition of NeuroScios, a CRO specialized in clinical studies in Alzheimer’s, strengthens our alignment with ATx’s therapeutic focus even further,” he said.
Arctic Therapeutics was established in 2015 as a spin-off from the US-based Center for Applied Genomics (CAG), a research center at the Children’s Hospital of Philadelphia led by the company’s founder Hakon Hakonarson.




 Freepik.com
Freepik.com