AMT-130 gets FDA breakthrough therapy designation
Amsterdam-based uniQure NV’s gene therapy candidate AMT-130 has been granted FDA Breakthrough Therapy Designation for the treatment of the neurodegenerative Huntington’s disease.
The FDA has granted AMT-130 Breakthrough Therapy designation, following Fast Track designation (2017), Orphan Drug designation (2019) and most recently Regenerative Medicine Advanced Therapy (RMAT, 2024) from the recently shrinking US agency. Uniqure said it will submit a Biologics License Application (BLA) for AMT-130 by the second quarter of 2025.
The agency’s decision is based on clinical results from 13 patients (six low-dose, seven high-dose) enrolled in an ongoing placebo-controlled Phase I/II trial of AMT-130 for HD in the US. In July 2014, uniQure presented 24-month interim results showing a 30% dose-dependent slowing of disease progression on the Unified Huntington’s Disease Rating Scale (cUHDRS), a multidimensional measure of HD progression, and a statistically significant 11% reduction in the neurodegeneration biomarker NfL.
Breakthrough therapy designation is intended to accelerate the development and testing of investigational medicines that provide early clinical evidence that the medicine can significantly improve treatment compared to other available medicines.
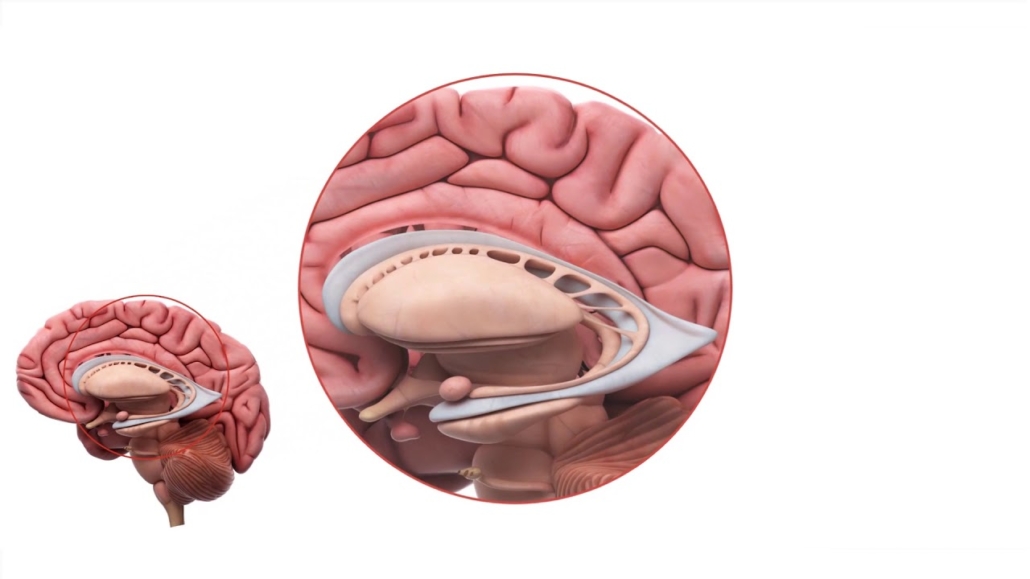
AMT-130, an AAV5-derived microRNA that targets HTT mRNA to reduce levels of mutant huntingtin (HTT) protein in the brain, is administered as a single infusion directly into the striatum of the brain through a surgical procedure. To date, a total of 45 patients have been treated with AMT-130 in the placebo-controlled US study and an open-label European study.



 Pharmahungary Group
Pharmahungary Group