AMR: GARDP and Entasis Therapeutics start pivotal study
Entasis Therapeutics Inc (Waltham, USA) and non for profit organisation GARDP (Geneva, Switzerland) have initiated Phase III testing of the novel antibiotic zoliflodacin to treat infections with the WHO priority pathogen N. gonorrhoea.
In the open-label study enroling 1,000 patients, safety and efficacy of zoliflodacin will be compared with a combination of azithromycin and ceftriaxone, the current standard of care. oliflodacin is a novel, first-in-class oral antibiotic being developed for the treatment of uncomplicated gonorrhoea. Following positive Phase II results previously published in the New England Journal of Medicine (NEJM), Entasis and GARDP have partnered to complete late stage development, with GARDP fully-funding and sponsoring the global Phase III trial.
Under the partnership agreement, GARDP is responsible for the Phase III trial and pharmaceutical development activities for zoliflodacin to support regulatory approval and market access and availability. GARDP has commercial rights to zoliflodacin in up to 168 low- and select middle-income countries, while Entasis retains commercial rights in the rest of the world.
The trial is expected to enrol approximately 1,000 adults with urogenital gonorrhoea from clinical trial sites in the United States, Netherlands, Thailand and South Africa. Patients included in the trial will be randomized (2:1) to receive either zoliflodacin or a combination of ceftriaxone and azithromycin and will be assessed one week later for persistence of the infection. Data from the phase III clinical trial is anticipated in 2021.
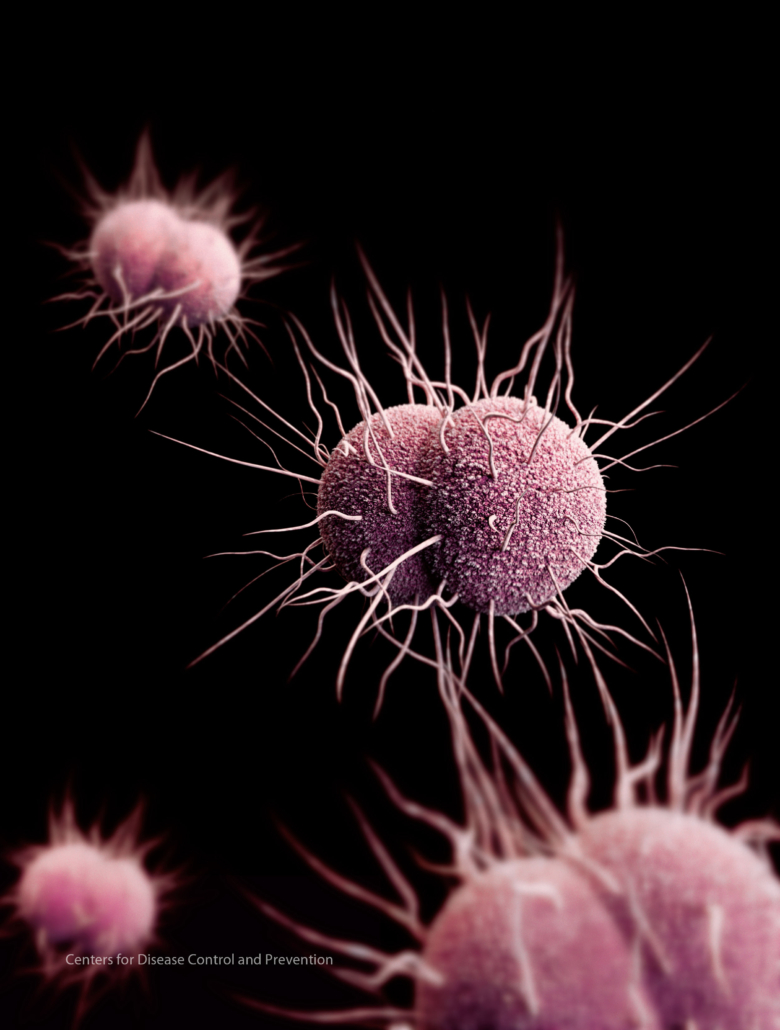
Gonorrhoea is a common sexually transmitted infection (STI) affecting both men and women, particularly between 15 and 24 years old.Globally the infection rate of gonorrhoea is increasing, with 87 million new cases estimated each year. Uncomplicated gonorrhoea infections carry high morbidity, enhance transmission of other sexually transmitted diseases and are highly stigmatized. Gonorrhoea is caused by the bacterium Neisseria gonorrhoeae, which has progressively developed resistance to globally-recommended treatments and has been identified by the World Health Organization as among a family of priority pathogens’ posing the greatest threat to global health.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink