Addex further improves liquidity
Following a private placement worth CHF3m in February, Swiss allosteric modulator specialist Addex Therapeutics has increased its capital to finance clinical studies of dipraglurant in Parkinson's and ADX71441.
Addex announced that it has increased its capital through the issue of 1,930,435 new registered shares at nominal value of CHF1 each to Addex Pharma S.A, its 100% subsidiary. The transaction has been executed to provide the group with additional treasury shares that can be used in the future to raise funds in an efficient manner.
Earlier this year, the company boosted its cash position from CHF1.4m at December 31, 2016 to CHF3.9m through sale of 1,623,427 treasury shares for gross proceeds of CHF3,282,093. According to Tim Dyer, Addex’ latest transaction has been executed to provide us with additional financing flexibility as we execute on our strategy to advance the clinical development of dipraglurant and ADX71441.
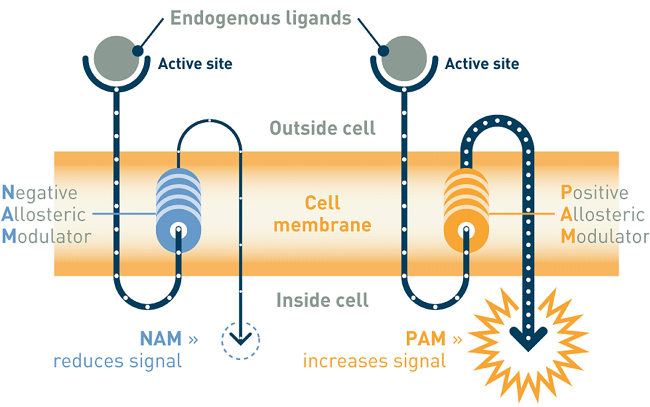
Addex’ lead drug candidate, dipraglurant, an oral mGluR5 negative allosteric modulator, has completed a Phase IIa dose escalation study in 76 patients with Parkinson’s disease levodopa-induced dyskinesia. Results published in September 2016 (https://www.ncbi.nlm.nih.gov/pubmed/27214664) showed acceptable toxicity and no worsening of parkinsonism. The company, however, said that the drug’s efficacy in reversing levodopa-induced dyskinesia warrants further investigations in a larger number of patients. Dipraglurant is being prepared to enter registration trials for PD-LID with support from the Michael J. Fox Foundation for Parkinson’s Research (MJFF). In parallel, dipraglurant’s therapeutic use in dystonia is being investigated with support from the Dystonia Medical Research Foundation (DMRF).
Addex’ second clinical programme, ADX71149 (mGluR2 positive allosteric modulator or PAM) is being developed in collaboration with Janssen Pharmaceuticals, Inc for epilepsy. In addition, ADX71441 (GABAB receptor PAM) has received regulatory approval to start Phase I and is being investigated for its therapeutic use in Charcot-Marie-Tooth Type 1A disease (CMT1A), cocaine and alcohol use disorder and nicotine dependence.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink