With many complex factors affecting a therapeutic candidate’s chances of approval, partnering with a qualified service provider can be the most effective way to produce material for clinical trials. Outsourcing allows you to delay critical capacity decisions until the fate of your molecule is more certain but, how do you ensure this approach results in a successful process that’s delivered within your target timeline?
ADVERTISEMENT
The current debates regarding vaccine production and logistics have also made it clear even to the layman: Pharmaceutical production depends on cold. Temperatures below -80?°C are just as common as the requirement to freeze precursors such as blood plasma within a specified time period. Refrigeration specialists with pharmaceutical know-how are in demand for these challenging tasks.
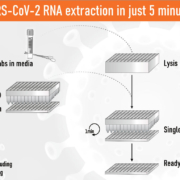
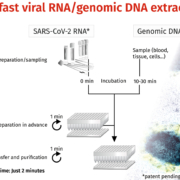
Although innovative biotechnologies are developed continuously, the method of nucleic acid extraction stayed unchanged for several decades. The classical bind-wash-elute approach seemed sufficient, but the COVID-19 pandemic uncovered the need for a new technique to be able to process multiple samples fast and in parallel. BioEcho developed a completely new technology, which simplifies and speeds up the process of RNA extraction dramatically.
Together with star investor Kleiner Perkins, Origenis GmbH, a privately held German biopharmaceutical company, successfully establishes its new business model for the clinical development of its neuroprotective drug pipeline. The formation and financing of Neuron23 Inc. was announced in South San Francisco.
The BioXp system is the world’s only automated platform for on-demand DNA assembly and amplification.
The Vienna Business Agency has set up affordable and flexible Startup Labs at the Vienna BioCenter. This will speed up the evaluation and implementation of new business ideas in biotechnology by the next coming generation of bio-entrepreneurs. Key assets include close proximity to outstanding academic research and highly successful innovators as well as access to cutting-edge research infrastructure.
Commemorative publication for the 25th anniversary of the IZB presents 70 profiles and visions of successful biotech start-ups. The 360-page publication describes the development of the Innovation and Start- up Center for Biotechnology into the leading biotechnology center in Europe
CDMO Continuous growth and diversification of the biotechnology market for pharmaceutical production have been recorded in the past years. It is expected that both trends, growth and diversification from blockbusters towards niche products, will continue. What requirements are derived from customers’ point of view and what are the advantages of Richter-Helm as a successful CDMO to fulfil such needs?
BioEcho develops single-spin and 96/384-well kits based on the patent pending single-step EchoLUTION nucleic acid extraction process. Highly superior to the commonly used silica-based solutions (bind-wash-elute), the isolated inhibitor-free RNA/DNA is detected with increased sensitivity. Furthermore, the whole procedure is up to 20-fold faster: Washing steps are not required, saving time, costs and plastic waste. Due to the expertise of its founders and employees, BioEcho also uses its know-how to support diagnostic or pharma companies with specialized scientific knowledge. Especially in times like these, BioEcho helps to accelerate accurate tests on certain RNA/DNA viruses such as SARS-CoV-2.