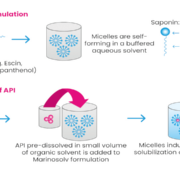
Therapies for autoreactive immune disorders have greatly advanced in the past decades but often have significant side effects. Marinomed Biotech AG strives to improve therapies by increasing the solubility and bioavailability of existing drugs. A proprietary pipeline and Solv4U, a B2B offering for API solubilization, enable the development of clinically meaningful new or improved therapies.
ADVERTISEMENT
Most people know Switzerland for its watches, mountains, and chocolate. But biopharma professionals know that this country in the heart of Europe offers much more: the ideal setting for innovation and production in the fields of pharma and biotech.
The CRO industry is predicted to grow considerably over the next five years. Trials are becoming more complex, and technology is becoming increasingly interwoven with processes. The CRO of tomorrow must be tech smart, agile, and environmentally conscious to remain relevant. In this changing landscape, how does a CRO cut through the noise in 2022-30?
nearly 200 participants
from 23 countries
30 posters
28 talks
Why are so many vaccine developers choosing Thermo Scientific Charged Aerosol Detection (CAD)? Hear the third very good reasons why CAD was used for analysis of lipid nanoparticles in approved mRNA vaccines.
Nanobodies are emerging as important tools for tumor diagnosis and treatment due to their small size, simple humanization, low immunogenicity, superior affinity and stability, high penetration, and adequate levels of solubility, and are expected to revolutionize the antibody-based drug therapy. In 2018, the world´s first nanobody was approved for marketing in the European Union to treat adult patients with acquired thrombotic thrombocytopenic purpura. At present, more than 20 nanobodies are being tested in clinical research worldwide, mostly in clinical phase I or II.
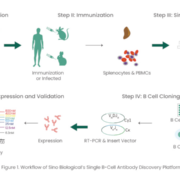
Since the approval of Orthoclone OKT3 in 1986, more than 100 monoclonal antibodies (mAbs) have been approved by the Food and Drug Administration (FDA) to treat a variety of diseases ranging from autoimmune disorders, infectious diseases, and cancer [1-2]. In the context of the current COVID-19 pandemic, it´s crucial that these biologics are developed rapidly and efficiently. Among various antibody discovery approaches, including hybridoma technology, single B cell screening is a powerful and efficient strategy for generating antigen-specific mAbs based on the direct amplification of the VH and VL regions encoding genes from single B cells [3-4]. Notably, single B cell screening has various advantages that include maintaining the naïve VH/VL pairing, requiring relatively few cells, and the ability to discover antibodies against challenging targets.
ACHEMA is the world forum and leading trade show for the chemical, pharmaceutical and food industries. It’s where new developments are unveiled and trends are discussed.
Successful restart: From 31 May to 1 June 2022, Chemspec Europe attracted 5,122 industry experts from 57 countries, of which 3,162 were trade visitors. A total of 287 exhibitors from 26 countries presented their product portfolio on a net exhibition space of 5,300 m2 at Messe Frankfurt, Germany. With topics such as sustainable innovations, regulatory compliances, circular bio-economy, green chemistry, digitalisation, and the flourishing startup scene, the 35th International Exhibition for Fine and Speciality Chemicals highlighted insights of current and future developments within the industry.
Amidst the rapid evolution of DNA sequencing technologies in past years, the industry has been working tirelessly to make genomics more accessible. Since the Human Genome Project, which saw scientists from six countries coming together to map the first human genome at nearly $3 billion over 13 years, the cost of whole-genome sequencing has fallen to less than $1,000 today. Yet, the quest to produce larger amounts of short- and long-read data in a shorter span of time and at an even lower cost continues.