The European Commission has approved Amgen’s Uplizna in generalized myasthenia gravis, moving the big biotech into a market served by argenx, Johnson & Johnson and UCB.
ADVERTISEMENT
French biotech GENFIT will receive a $20 million commercial milestone payment after Ipsen reported $208 million in full-year 2025 net sales of Iqirvo in primary biliary cholangitis, surpassing the $200 million threshold set out in the companies’ 2021 licensing agreement.
Europe produces world-class life sciences research, yet still struggles to finance and scale it. Venture capital in European biotech accounts for just 7 per cent of global funding, while 66 of the 67 EU biotech companies that went public over the past six years chose to list outside Europe. Capital is available globally; Europe is struggling to capture it.
Chemify has struck a deal with Zeon, securing investment from the company’s venture capital wing and signing up to make novel compounds and materials for its new partner.
A surprise move from Darmstadt to Paris: outgoing Merck CEO Belén Garijo is set to take the helm at French pharmaceutical group Sanofi after Paul Hudson unexpectedly failed to secure a contract extension from the board.
French biopharma THX Pharma has signed a strategic licensing deal with Biocodex, an independent French pharmaceutical group, to advance two drug candidates in three rare genetic disorders with high unmet need.
Cambridge-based Microbiotica’s ulcerative colitis (UC) candidate met its primary and secondary phase 1b study objectives on safety, efficacy signals and bacterial engraftment. The company has reported positive results from a small, placebo-controlled study of its oral precision microbiome therapy MB310 in patients with mild-to-moderate ulcerative colitis, adding a rare positive clinical signal in a disease area where microbiome approaches have so far struggled to gain traction.
Zurich-based oncology biotech Araris Biotech AG has entered into a research collaboration with an option to license with Japan’s Chugai Pharmaceutical, a subsidiary of Roche. The aim of the partnership is to develop next-generation antibody–drug conjugates (ADCs). While Araris itself is now Japanese-owned – a wholly owned subsidiary of Taiho Pharmaceutical, part of the Otsuka Group – its research activities remain firmly rooted in Zurich. That presence was highlighted last year when the Strüngmann brothers, German billionaire twins, awarded a prize to the company’s founders.
The Australian–Swiss plasma specialist CSL is relying on technology from Switzerland for recombinant polyclonal immunoglobulins (IgG). With Memo Therapeutics, the company has entered into a collaboration and option agreement with a total potential value of up to CHF 265 million. This is good news for Memo, while CSL, following substantial job cuts including in Marburg, could also use some different headlines for a change.
After more than a decade of failed drug development efforts, osteoarthritis remains one of biopharma’s most stubborn challenges. Despite affecting more than 600 million people worldwide, no therapy approved in Europe or the United States has yet been shown to slow or modify disease progression. Against that backdrop, 4Moving Biotech (4MB) is betting that a familiar drug class, GLP-1 receptor agonists, can succeed where others have fallen short.





 SANOFI
SANOFI
 Microbiotica
Microbiotica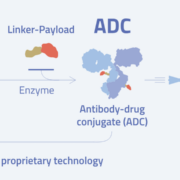 Araris Biotech AG
Araris Biotech AG Freepik.com
Freepik.com