German infection specialist InflaRx (Jena) raised US$55m in a Series D financing co-led by Bain Capital Life Sciences LP, Cormorant Asset Management LLC and RA Capital Management LLC to finance its clinical programmes with its lead asset IFX-1.
ADVERTISEMENT
Norwegian Targovax ASA (Oslo) has reported tremendous safety, immunogenicity, and one-year survival data, of in the modified cohort of the TG01 trial in resected pancreatic cancer patients.
Sanofi is investing €170m to expand its vaccine manufacturing site in Val de Reuil, France, which produces 900 million vaccine doses worldwide each year.
British researchers have identified interleukin 36 (IL-36) as a druggable proinflammatory driver of psoriasis. Because of its very targeted effects of antibodies directed against the cytokine they want to start clinical tests in man.
German-Israeli Neurim Pharmaceuticals announced top line efficacy results of its insomnia drug PedPRM in children with autism spectrum disorders (ASD).
French cancer drug encapsulation specialist Erytech Pharma SA wants to raise US$100m (€85m) in an IPO at NASDAQ and a subsequent private placement outside Northern America to finance pivotal trials of eryaspase in ALL and pancreatic cancer.
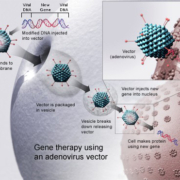
As first gene therapy ever, AMT-130 has been granted FDA Orphan Drug status in Huntington’s Disease.
Promethera Biosciences and Shibuya collaborate to establish a breakthrough cell therapy manufacturing platform.
Cancer immunotherapy specialist Immatics Biotechnologies GmbH (Tuebingen, Germany) raised US$58m in a Series E financing round led by German investors dievini Hopp BioTech Holding and AT Impf as well as Wellington Partners and new investor Amgen.
Scotch cancer corp NuCana plc was up 3% (to US$18.61) after closing an initial public offering on Friday led by Citigroup, Jefferies and Cowen and co-managed by William Blair.