A Phase III study (IMpower150) sponsored by Roche has shown that a combo of the company’s PD-L1 blocker atezolizumab (Tecentriq) and VEGF blocking antibody bevacizumab (Avastin) plus paclitaxel + carboplatin vs Avastim plus chemotherapy significantly reduced the risk of disease progression or death in patients with advanced lung cancer.
ADVERTISEMENT
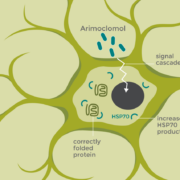
Danish heat shock protein specialist Orphazyme raised €80m+ through an IPO at Nasdaq Copenhagen adding momentum to the current upswing of European biotech.
The BEAM Alliance has called out worldwide stakeholders to support SME-driven innovation to revive the product pipeline fighting antimicrobial resistance (AMR). In a position paper the alliance representing roughly a fifth of the global 250 antibiotics developers describes what SMEs need to make their 100 pipeline drugs a success in fighting AMR.
German Bayer AG has acquired ex-US commercialisation rights to Loxo Oncology Inc’s Phase II lead larotrectinib (LOXO-101), which targets TRK (tropomyosin receptor kinase) fusions in 17 cancer types, and LOXO-195, a Phase I/II compound designed to prevent acquired resistance against larotrectinib.
Billionaire Bill Gates announced he is going to invest US$50m private money into the Dementia Discovery Fund (DDF) to support fresh ideas for developing Alzheimer drugs and diagnostics. The venture capital fund, which kicked off in 2015, includes investors such as GlaxoSmithKline, Johnson & Johnson, Eli Lilly, Pfizer and Biogen Idec as well as the UK government.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) recommended seven medicines for approval, including two orphan medicines, plus three generics at its November 2017 meeting.
More than 4,000 participants from all over the globe visited Berlin in early November to take part in this year’s BIO-Europe. The event continues to be on the upswing – like the biotech industry itself.
Conversion of agricultural waste into useful products is a worldwide need. As part of the European Project Valor Plus, Spanish biotechindustry association ASEBIO invited its members to visit the recently opened second-generation biorefinery Clamber.
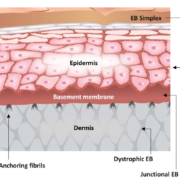
For the first time researchers at universities Bochum (Germany), Salzburg (Austria), and Modena (Italy) have shown that autologous transgenic keratinocyte cultures can regenerate an entire, fully functional epidermis on a seven-year-old child suffering from junctional epidermolysis bullosa (JEB).
German inflammation and autoimmunity specialist InflaRx AG (Jena) announced it will offer 6,667,000 common shares at an Nasdaq initial public offering price of $15.00 per common share, resulting in total gross proceeds of approximately US$100m.