A new method to test the likelihood of a drug turning into a potentially harmful version of itself when it enters the body has been developed by researchers at Cardiff University.
ADVERTISEMENT
Japanese Pharma major Takeda had announced its intention to take over Belgian adipose stem cell therapy specialist Tigenix, which has already exclusively licenced its lead product darvadstrocel (Cx601) to treat Crohn’s disease. In December darvadstrocel received a recommendation from the European Medicines Agency to market the product in Europe in patients with complex perianal fistulas, one of the most disabling manifestations of Crohn’s disease.
German human and veterinay medicines major Boehringer Ingelheim has more than doubled the amount in funding of its Venture Fund (BIVF) to €250m. Additionally, the investment focus has been extended to include infectious diseases and digital health.
Swiss allosteric modulation specialist Addex Therapeutics has licenced its GABA modulator ADX71441 to US addiction therapeutics expert Indivior plc.
A triple receptor agonist developed for diabetes could be used to treat Alzheimer’s after scientists found it "significantly reversed memory loss" in mice through a triple mode of action.
There is a lot of reports on technologies that have already entered the mainstream and will continue to grow in 2018 such as T-cell receptor targeted cancer, autoimmune and gene therapies. European Biotechnology Magazine takes a look on what really is new and has the potential to change current medical paradigms.
Therapy-induced senescence (TIS), a lasting chemotherapy-evoked proliferative arrest of tumor cells, has been thought to be irreversible. As it turns out now, it makes tumour cells, which survive, more aggressive and worsens prognosis.
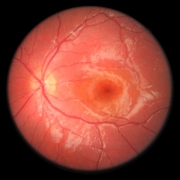
Scientists have generated sheets of human eye cells arranged on a biological scaffold, which they used to successfully treat vision disorders in rats.
The European Commission has closed its very first infringement procedure under the Penalty Regulation. During an inspection at Roche’s UK headquarters in 2012 the British MHRA found data on 80,000 not properly reported adverse events of approved Roche meds, 15,161 of which included deaths.
Hadean Ventures announced the launch of Hadean Capital I, a fund that will invest up to € 100 m in life science companies, with a particular focus on the Nordic region.