Shortly before the Global Bioeconomy Summit (19.-20.4.2018) in Berlin will begin, Germany’s new Research Minister Anja Karliczek announced three major initiatives to strengthen the biologisation of the industry and the foundation of innovative biotechs.
ADVERTISEMENT
Mid last year, the company Pierre Guerin has opened a new location in Germany, Hildesheim.
British drug major GlaxoSmithKline (GSK) has passed its orphan autologous cell and gene therapy pipeline including its EU-approved ADA-SCID treatment Strimvelis to British/US Orchard Therapeutics. GSK acquired a 19,9% equity stake in Orchard and a board membership.
A public offer from orphan drug major Alexion Pharmaceuticals Inc. has been accepted by Wilson Therapeutics‘ Board of Directors. Alexion’s SEK232/share bid would total in an acqusition price of SEK6.564bn or €638.8m.
Swiss drug major Novartis has secured a potential stake in the multibillion dollar market of treatments for the orphan genetic muscle disease spinal muscular atrophy (SMA). The company acquired AveXis Inc, whose SMA gene therapy AVXS-101 is in Phase III testing.
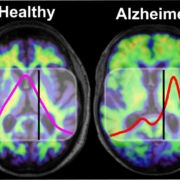
A newly developed blood test indicates Alzheimer’s disease on average eight years before the clinical diagnosis.
Human drug trials are compromised by poor reporting and potential bias of animal research. German researchers analysed more than 700 preclinical animal studies and investigator brochures provided by three medical centres. They call to establish documentation standards for IBs/preclinical dossiers.
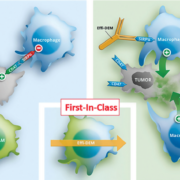
French Ose Immunotherapeutics SA has licenced global commercialisation rights of its preclinical programme OSE-172 to Boehringer Ingelheim which hopes to complement its immunoncology portfolio with a tumour microenvironment modifier that reactivates effector T cell responses.
French allogenic CAR-T cell therapy developer Cellectis is set to launch an underwritten public offering of $175m of its American Depositary Shares (ADS), each representing one ordinary share of Cellectis.