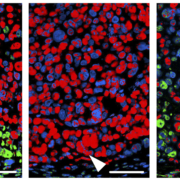
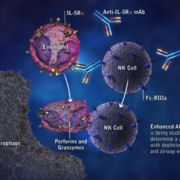
Researchers at LMU Munich have unraveled a new strategy of colon cancers to evade targeted therapies.
ADVERTISEMENT
CAR-T cell therapy specialist Kite Pharma, now part of Gilead Sciences, has expanded its cell therapy manufacturing footprint in Europe. A 117,000 square-foot facility in Hoofddorp, the Netherlands, will be operational by 2020.
CAR-T cell developer bluebird bio Inc. has extended its potential US$1bn deal with cancer neoantigen TCR specialist MediGene by two new targets adding US$500m potential revenues to the licence agreement.
British drug maker AstraZeneca’s IL-5Ralpha blocker benralizumab did not reduce exacerbations significantly compared to placebo in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
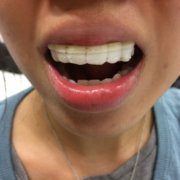
A research team headed by Jean-Christophe Leroux from ETH Zurüch has created the first-ever 3-D printed mouth guard that could deliver drugs to the wearer and is highly customizable, in terms of drug release and design.
Following weeks of negotiations and four rejected bids, British neurology and orphan drug giant Shire has agreed to Takeda’s latest take-over offer of £46bn (€52,38bn).
Vas Narasimhan, the newly appointed CEO of Swiss pharma giant Novartis, introduced himself to the Swiss biotech industry with a stimulant talk at the Swiss Biotech Day. Apart from that participants of the annual gathering were in high spirits as the Swiss biotech sector continues to grow steadily.
Swiss injection pen developer Haselmeier and Boston-based Common Sensing have partnered to develop smart connected self-injection devices that can monitor drug administration, dosing and efficacy of therapies.
The UK-based pioneer in marine biotechnologies Jellagen has closed a £3.8m funding round to boost sales of its next-generation jellyfish-based medicine. Lead investor were Newable Private Investing and the Development Bank of Wales.
Iterum Therapeutics plans to raise up to US$92m in an initial public offering on US technology stock market Nasdaq. The Irish company develops oral and IV antibiotics for urinary tract infections and intra-abdominal infections.