ADVERTISEMENT
European stock exchange operator Euronext kicked off its fourth annual capital markets training programme for non-listed companies – this time also including companies from Germany, Italy, Spain, and Switzerland.
Novo Nordisk A/S (NOVOB:DC) said it plans to restructure its entire Research & Development (R&D) organization. Consequently, the company will lay off 400 employees from R&D roles in Denmark and China.
The FDA has requested additional clincal data demonstrating that Pharming Group’s recombinant C1 esterase blocker Ruconest can prevent hereditary angioedema attacks (HAE).
CRISPR Therapeutics AG announced a development partnership with ViaCyte Inc to develop genome-edited pancreatic islet cells, which do not require immunosuppression, to treat diabetes.
Positive Phase 3 results with Sanofi/Regeneron‘s IL4/IL-13-targeting antibody dupilumab (Dupixent) in adolescents with moderate-to-severe atopic dermatitis will form the basis of a regulatory submission in this new patient population, according to announcements of Sanofi.
Slowing the leak of Calcium leak into cardiomyocytes does not improve the pumping malfunction in heart failure, German cardiologists report in Science Translational Medicine.
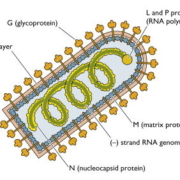
EMBL Ventures has announced that its portfolio company ViraTherapeutics is set to be acquired by Boehringer Ingelheim in a €210m million (US$245m) transaction.
Orphan drug leader Shire plc has been granted EU marketing authorization for its recombinant van Willebrand factor (rVWF) vonicog alfa as second-line treatment for van Willebrand disease, the most common blot clotting disorder..
Drug Developers dealing with PD1-PDL1 checkpoint inhibitors must consider new safety problems associated with the hyped cancer meds.