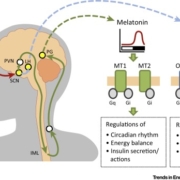
Sosei Group Corporation has spun out two programmes of its neurology pipeline into two newly created companies funded by venture fund Medicxi.
ADVERTISEMENT
EMA’s human medicines committee (CHMP) recommended six medicines for approval at its January 2019 meeting.
Hitachi Chemical Co. Ltd. is set to takeover cell and gene therapy CDMO apceth Biopharma GmbH to get a foothold in Europe, the second largest market for advanced therapies after the US.
Dewpoint Therapeutics has attracted interested of investors with a completely new approach that might cure a huge array of devasting diseases.
Marinomed has set the offer price for its IPO at Vienna Stock Exchange at the lower limit of €75 per new share.
Novozymes has entered into a partnership with French Carbios and its subsidiary Carbiolice, which aim to launch degradable bioplastics with limited lifespan for the packaging industry by 2020.
A new assay that accurately measures how arthritis patients respond to treatment with TNFalpha blockers such as adalimumab, open up the way to predict efficacy.
In order to incentivise drug companies to re-enter development of antibiotics, the UK government announced it will establish a licence model to assure developers sufficient return on investments (ROI).
Immunoncology and vaccine specialist BioNTech AG is broadening its antibody discovery capabilities by acquiring MAB Discovery‘s antibody generation unit.
Swiss fine chemicals specialist Clariant has hammered out a R&D pact with ExxonMobil and Renewable Energy Group (REG) Inc to combine technologies in order of producing biodiesel from cellulose