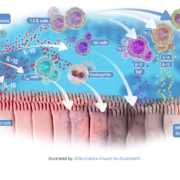
The global antibacterial innovation network CARB-X has expanded its reach by adding six new life-science organisations to its fight against antimicrobial resistance (AMR).
ADVERTISEMENT
Swiss pharma giant Roche has complemented its drug pipeline in the lucrative hemophilia A market by taking over hemophilia A gene therapy developer Spark Therapeutics for US$4.3bn.
Sodium chloride can promote immune responses that have been linked to atopic dermatitis. Researchers have now unraveled the mechanistic details between salt intake and activation of T cells.
Dutch autoimmunity specialist Calypso Biotech BV has raised €20m in a Series A financing co-led by Gilde Healthcare and Inkef Capital and co-financed by Johnson & Johnson Innovation – JJDC, Inc. (JJDC), and the company’s seed investor M Ventures.
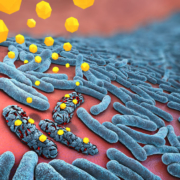
Evotec AG and the Helmholtz Centre for Infection Research (HZI) have joined platforms in order to find novel resistance-breaking antibiotics against Gram-negative bugs.
Researchers for the first time have found a way to prevent immune rejection of allogenic iPSC-derived cell and tissue transplants.
Swiss newco Arvelle Therapeutics has baged a $180m (€159.1m) series A financing led by LSP. The company licenced the anti-epileptic candidate cenobamate (YKP3089) for $100m (€88.4m) upfront from SK Biopharmaceuticals.
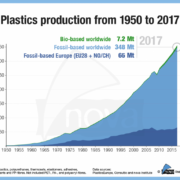
The market for bio-based building blocks and polymers showed moderate growth to 7.5 million tonnes in 2018 and will grow with a CGAR of 4% by 2023 reports German nova-Institute.
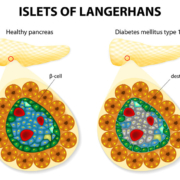
Swiss researchers have demonstrated a way to cure type I diabetes without cell transplantation. In model mice the reprogrammed non-islet cells from human pancreas
French industrial biotech company Afyren announced a capital increase of €21m in a financing round led by Sofinnova Partners and Valquest Partners to scale up its biobased organic acid production to industrial scale.