ADVERTISEMENT
Researchers have discovered that crosstalk of a newly discovered stem cell population with quiescent stem cells may help repair teeth in the future.
Bayer has widened its pipeline in neurology, cardiology, and immunology by acquiring induced pluripotent stem cell specialist BlueRock Therapeutics.
Using a platform tailored to patients’ specific cancer mutations, British and US researchers for the first time detected residual disease in breast cancer patients.
SillaJen, the licensor for French immunoncology specialist Transgene SA, has stopped enrolment into the Phase III Phocus trial after an Independent Data Review Comittee predicted the study endpoint won’t be met.
Wellington Partners has closed its largest Life Sciences Fund ever. The VC specialist outperformed its intial target of raising €120m to €150m and closed at €210m.
Venture Captital specialist LSP led a €67m Series A financing in peptide developer Alizé Pharma 3.
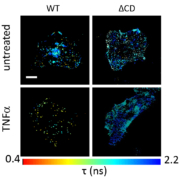
Two research teams have unraveled the complex dynamics of TNF? receptor signaling and have identified compounds that may be safer than current treatments.