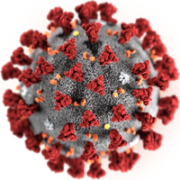
The Innovative Medicines Initiative (IMI) has announced a €45m funding for the development of therapeutics and diagnostics to tackle coronavirus outbreaks.
ADVERTISEMENT
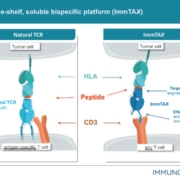
With the financing led by General Atlantic, the British company wants to expand a novel platform and advance its ImmTAX pipeline to the clinic.
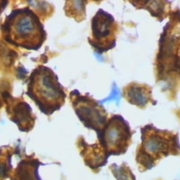
High amounts of injected vitamin C may delay the growth of many types of tumours and boost the anticancer power of immune checkpoint therapies, report Italian oncologists.
Roche AGs has paid US$30m upfront in a potential US$1.7bn deal to get access to Bicycle Therapeutics plc’s technology platform to target immune checkpoints.
Negotiations of the EU Council on the EU’s seven-year budget have failed, putting R&D funding at risk for significant cuts.
Royal DSM NV has entered the growing market of human milk oligosaccharides (HMO) taking over the producer of recombinant 2′-fucosyl lactose, Danish Glycom A/S.
US-German immunooncology specialist Immatics Biotechnologies GmbH has licensed two of its TCR-T programs to GlaxoSmithKline for US$50m upfront.
Univercells S.A. cashes in up to €50m from the KKR subsidiary Gamma Bioscience to advance its gene and cell therapy manufacturing platform.
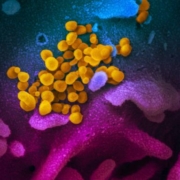
Shortly before a EU health ministers discuss the spread of the Chinese coronavirus, EU diagnostic labs have considered themselves prepared for a possible Sars-CoV-2 epidemic.