ADVERTISEMENT
Swedish Cellink AB has announced a bid of $110m to take over all shares of Discover Echo Inc., 11% of which to be paid in newly issued shares.
Major CDMO Catalent has swallowed German RheinCell Therapeutics GmbH, an iPSC specialist, to expand its offerings in cell and gene therapies.
In an effort to ban AI tools for facial recognition, the EU risks to block European medical geneticists to recognise genetic diseases.
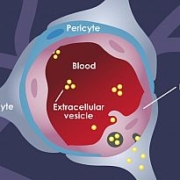
NeuVasQ Biotechnologies has raised €20m through a Series A investment to advance its technology platform that restores the integrity of the blood-brain barrier in acute neurological disorders.
Coriolis Pharma will launch operations at its currently expanded ATMP development facilities by Q4/2021.
Company seeks new opportunities for use of mRNA therapeutics.
U.S. government to purchase at not-for-profit price 200 million doses in 2021 and 300 million in the first half of 2022.
Funding to accelerate clinical progression of Iksuda’s pipeline of new generation of antibody drug conjugates, targeting tumours with high unmet need.