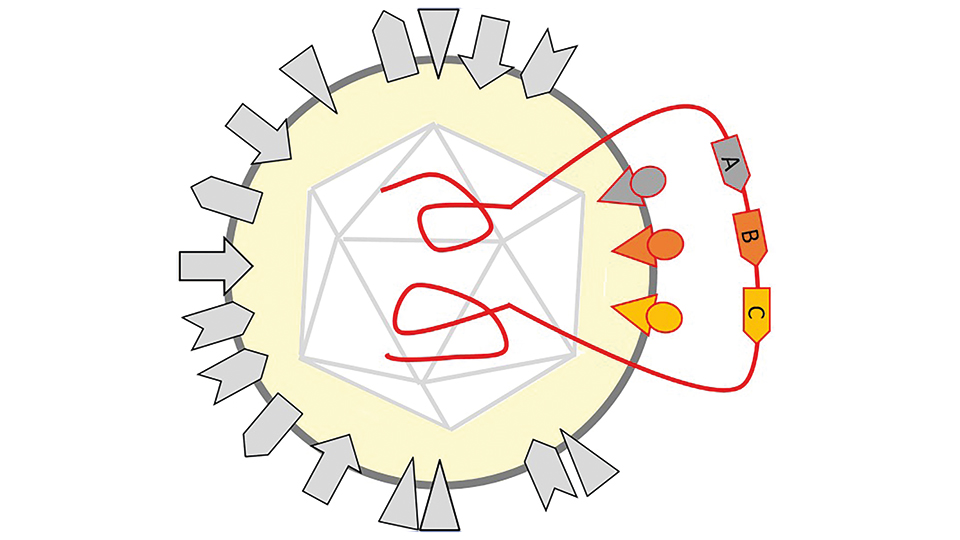
TheraVision – an oncolytic virus platform technology
Since virus immunotherapies have the potential to improve therapy responses, we established a platform technology based on Herpes Simplex Virus Type 1 (HSV1). TheraVision constitutes a complete development pipeline including a proprietary HSV1 engineering vector for the modular integration of transgenes, a GMP-compliant production process, and complex preclinical test systems including human tumour and immune cells to demonstrate sensitivity and safety.
Through genetic modification, a proprietary HSV1 platform vector has been established using the Bacterial Artificial Chromosome technology. This engineering platform that was filed for patent allows for a modular and versatile programming of the HSV1 genome by facilitated integration of transgenes as well as high-capacity engineering, and is thus highly suited as research tool.
A programmable oncolytic HSV1
Further modification of the vector is aimed at deleting neurotoxicity genes of HSV1. The resulting attenuated vector led to a significant reduction of the viral load in the mouse brain compared to an unmodified HSV vector demonstrating its safety. As proof of concept, oncolytic viruses (OVs) were developed to target the Non-Small Cell Lung Carcinoma (NSCLC). To this end, transgenes were implemented into the platform vector for optimized targeting to tumour cells and immune modification of the tumour microenvironment. The engineered OVs infected NSCLC cells specifically, virus-encoded immune checkpoint inhibitors were able to bind the receptors of the immune system and thus proved to be functional. Thus, transgenes for targeted virus-mediated oncolysis and immune cell-mediated therapy are being combined into a single viral vector, resulting in the efficient destruction of tumours and potentially even metastases.
Bioprocess development
A scalable pharmaceutical bioprocess for the production of HSV as active pharmaceutical ingredient (API) was developed. The manufacturing process is based on smart technologies through a combination of already established unit operations supported by bioinformatics modelling resulting in generic robustness as well as high yields, biological activity and purity. The process sequence was designed to meet GMP requirements and represents a generic virus production platform that is applicable to the manufacturing of other viral vectors as well.
Preclinical testing
In parallel, preclinical test systems containing both human tumour and immune cells were established addressing different endpoints: An established cell and tissue bank from cancer patients is the source for 2D cell lines and 3D organoid models, which enable initial efficacy testing in the presence of autologous immune cells. Complex 3D in vitro tumour models on a biological tissue matrix depict the pathological microenvironment of the tumour and allow for testing over several weeks. Complex humanized animal models report on the sensitivity of the OVs using a highly sensitive optical system, supplemented by immunological methods. Together, these preclinical test systems showed that the engineered OVs enable the specific infection and destruction of the tumour cells and specifically influence the immune system.
Thus, with TheraVision, a production process based on HSV1 has been established to offer a broadly applicable platform technology for tailored oncolytic virus- and immune-therapies. The contributing institutions provide core competencies to complete the entire production process of this oncolytic virus technology.
This article was originally published in European Biotechnology Magazine Winter Edition 2021.



 Unsplash+
Unsplash+