Serialisation: Time is running out
Drug makers have only until two years from now to make their drugs and packaging counterfeit-resistant. By 9 February, 2019, every prescription drug pack must carry a 2D data matrix code that can be tracked by wholesalers and pharmacists along each stage of the value chain. Additionally, each pack must be sealed with an anti-tampering device. If companies and the NMVOs that handle national databases can't manage the task, their drugs cannot be sold after the deadline.
According to a global surveillance and monitoring system implemented by the WHO in 2013 to assess the economic impact of counterfeit drugs, over 920 falsified medical products have been reported by member states thus far. While counterfeit drugs are leaking into the legal supply chain, it’s highly important to secure it from criminal action, says Dr Reinhard Hoferichter, spokesman for the board of securPharm. Germany’s National Medicines Verification Organisation (NMVO) is the very first early adopter of the EU’s Falsified Medicines Directive 2011/83/EG and its delegated regulation. The regulation, which must be implemented in the EU member states plus Norway, Iceland, and Liechtenstein by February 2019, decribes exactly what drug manufacturers and prescription med sellers must do to block stolen and repackaged drugs from leaking into pharmacies: print a unique identifier onto every prescription medicine, and seal drug packs with an anti-tampering device.
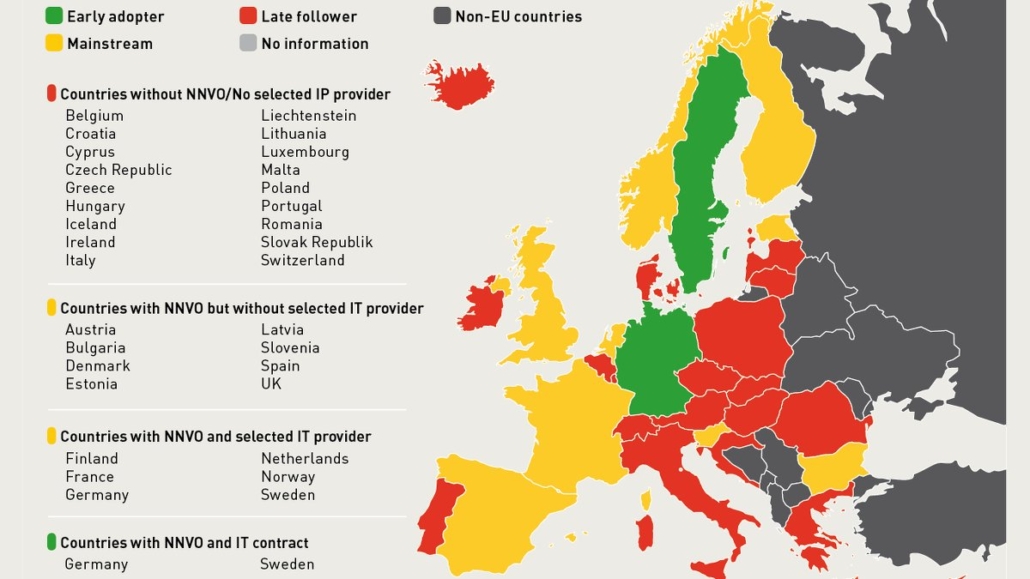
Currently, the EU sees two different speeds of implementation. Early adopters such as Germany and Sweden needed about two years to build their NMVOs with the players of the value chain and establish the technical system required for authentication of a drug. All other countries waited for the publication of the delegated act in February 2016.
According to the European Medicines Verification Organisation (EMVO), two-thirds of EU countries are lagging behind in implementation. The EMVO manages the EU data hub that will pool all data uploaded by NMVOs through their national blueprints to verify pharmacists and hospitals. By February, only 14 NMVOs had been incorporated and two IT providers for implementation had been selected, with Germany and Sweden taking the lead (see map). Four countries had not started technical preparations.
Intensive training required
The switch to the new serialisation and traceability requirements could become expensive, particularly for midsized companies. They not only must retrofit packaging lines and adapt manufacturing and IT systems to the new requirements, but also train operators, which comes on top of the annual EMVO/NMVO service costs based on the number of countries in which a drug is authorised. For small enterprises, this can be a substantial expenditure affecting product margins. For biopharmaceutical companies that have outsourced manufacturing and packaging, the challenge is to align their IT systems with those of their partners in order to appropriately transmit batch numbers etc. covered by the unique identifier. As securPharm’s system is in the advanced stage, 25% of German manufactures of Rx medicines have already joined it for practical testing. However, for most companies, it’s time to act now.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink