Lead optimisation – the sequence is the key
The efficacy of therapeutic antibodies is defined by their primary amino acid sequence. After discovery, antibody candidates often profit from protein engineering and sequence optimisation. Different technologies can improve the functional properties of antibodies as well as their biochemical and biophysical characteristics influencing manufacturing, process development, formulation, and other parameters of the final drug target product profile.
Therapeutic antibodies are the most important class of biopharmaceuticals, and they are indispensable for the treatment of many diseases. Fully human antibodies can be directly generated by different technologies such as in-vitro display technologies from libraries (phage, yeast, mammalian display) or by immunisation from transgenic animals endowed with human antibody genes or by both in combination. Selection and screening of early antibody candidates already define many properties of the final drug such as high target specificity and selectivity, or even special features such as cross-reactivity with animal models to facilitate preclinical development. However, it is unlikely, that early antibody candidates combine all favourable features in one molecule to already develop an optimal drug.
Lead optimisation
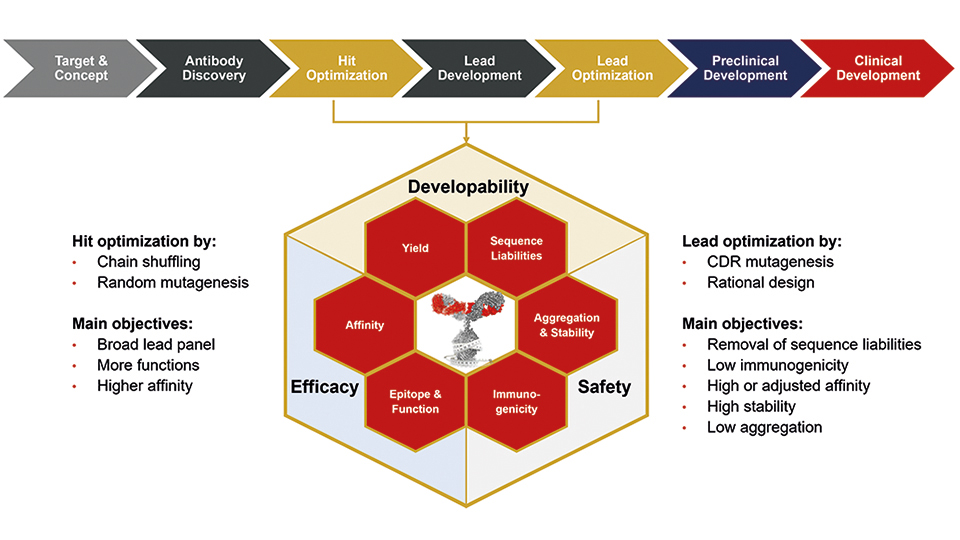
Hit and lead optimisation can help to improve functional properties of the antibody candidates such as affinity or stability, and they can also address issues concerning manufacturing or formulation relatively early in the development process. Therefore, it is important to consider and plan these optimisation steps at early stages of drug development, because they can save valuable time and high costs at the later development stages. Moreover, they de-risk the drug development programs before critical decision points, for example, before cell line development, when the antibody lead sequence is defined and cannot be changed anymore.
The exchange of single amino acids can strongly influence the functional properties, stability, expression levels, trend towards aggregation, and immunogenicity of an antibody, which means it can be decisive for the fate as a suitable therapeutic compound. Many properties are interdependent, for example higher stability can improve expression levels and can facilitate process and formulation development, or lower aggregation propensity may allow high-dose formulations, a prerequisite for subcutaneous administration route, and reduction of the likelihood of immunogenicity.
Hit optimisation soon after discovery can help to obtain larger lead panels with a broader range of functional properties to choose better early lead candidates. Here, random mutagenesis or chain shuffling can increase affinity and/or new functional properties, respectively. Top lead candidates often require additional protein engineering, which is tailored to the individual molecule. Bioinformatic approaches support many if not all steps of antibody development. Prediction of sequence liabilities does not only help to select or deselect lead candidates in the screening process, but also to identify sequence liabilities, which may interfere with later development steps. There is no one solution for all, but the combination of bioinformatical and molecular genetical technologies provide optimised solutions to improve almost any antibody molecule.
This article was originally published in European Biotechnology Magazine Spring Edition 2022.



 Unsplash+
Unsplash+