It is time to rethink biotech scaling
Biomanufacturing promises domestic, sustainable, sovereign and resilient production. Capacities are trapped in outdated scale-up processes. SPRIND urges investment and disruptive innovation to propel biomanufacturing. Demands are decentralised, non-sterile & continuous biomanufacturing capabilities.
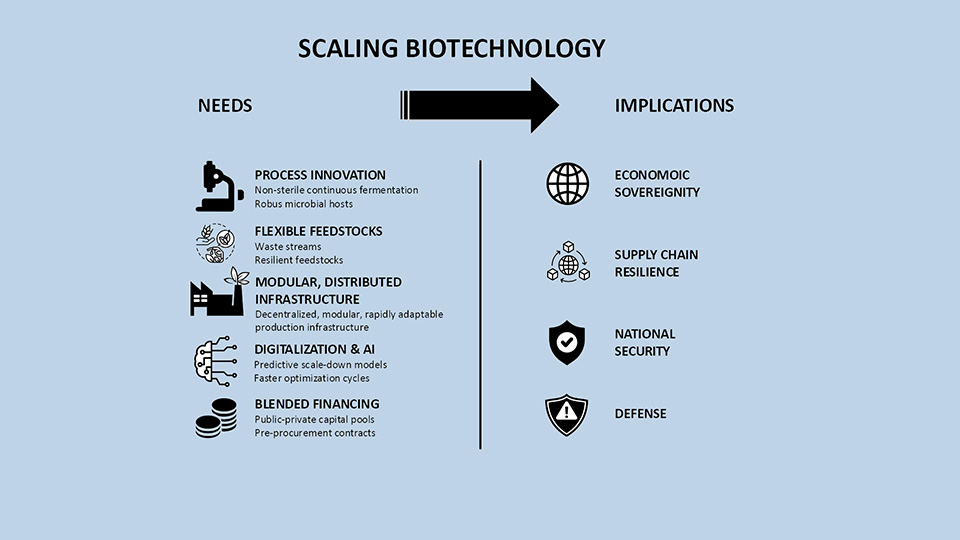
Biotechnology, empowered through advanced synthetic biology tools, has matured. And yet today’s capacity remains trapped in outdated scale-up processes. It still follows fine-chemical paradigms: sterile, low-volume, high-value production, locked into single feedstocks and products, with non-production chassis. This model fails to meet bulk industrial needs long promised but not yet delivered.
Pharma logic limits biotech
Fermentation processes require a rethink. Incremental innovations have sufficed for pharma but not for large-scale industrial biomanufacturing. The future lies in robust non-sterile fermentation, Needs and Impacts of biotech scaling robust hosts, and continuous fermentation processes. Scaled biomanufacturing must be feedstock-flexible, resilient, and incorporate scale-down models to better predict large-scale outcomes. The only certainty ahead for markets and manufacturing is unpredictability. Bioprocesses must be designed with industrial end-use in mind to avoid the infamous ‘valley of death‘ so many are unable to bridge. Digitalisation and AI can dramatically accelerate optimisation. Most challenges are engineering, not scientific; they can be solved in years, not decades. Sustainability is just one benefit; sovereignty and resilience are now paramount. In a shifting geopolitical landscape, domestic supply chains and production independence are critical for national security.
Security and sovereignty
Decentralised, modular, rapidly adaptable production infrastructure will strengthen resilience against pandemics, trade conflicts, and geopolitical shocks. SPRIND’s deep-tech bioeconomy strategy calls for targeted scaling infrastructure. Biotech, green chemistry, and traditional chemical engineering must converge to create integrated, hybrid value chains built for resilience. Regulatory frameworks must evolve. Genetic engineering should be treated as a tool for chemical manufacturing, not subjected to pharma regulations. Industrial biotech represents the next phase of the chemical industry and needs streamlined approval, flexible hybrid processes, and differentiated risk assessments. Rapid, unbureaucratic public funding, venture capital, tax incentives are required. SPRIND uses its procurement driven tools to unlock economic potential, exemplified by the Curricular Biomanufacturing Challenge. One initiative is not sufficient to drive change. Many more such initiatives are urgently needed to tackle the real challenges in biomanufacturing. Investments in biotech scaling are investments in future security, economic independence, and technological sovereignty.
This article from Dr Nicolas Krink, Senior Business & Research Analyst, German Federal Agency for Breakthrough Innovation SPRIND, Berlin, Germany, was originally published in European Biotechnology Magazine, Summer 2025.


 BIOCOM / aminul788 - Adobe Stock
BIOCOM / aminul788 - Adobe Stock adobe.stock.com - Cathy
adobe.stock.com - Cathy Afyren Group
Afyren Group