Council presses EC to decide on genome editing
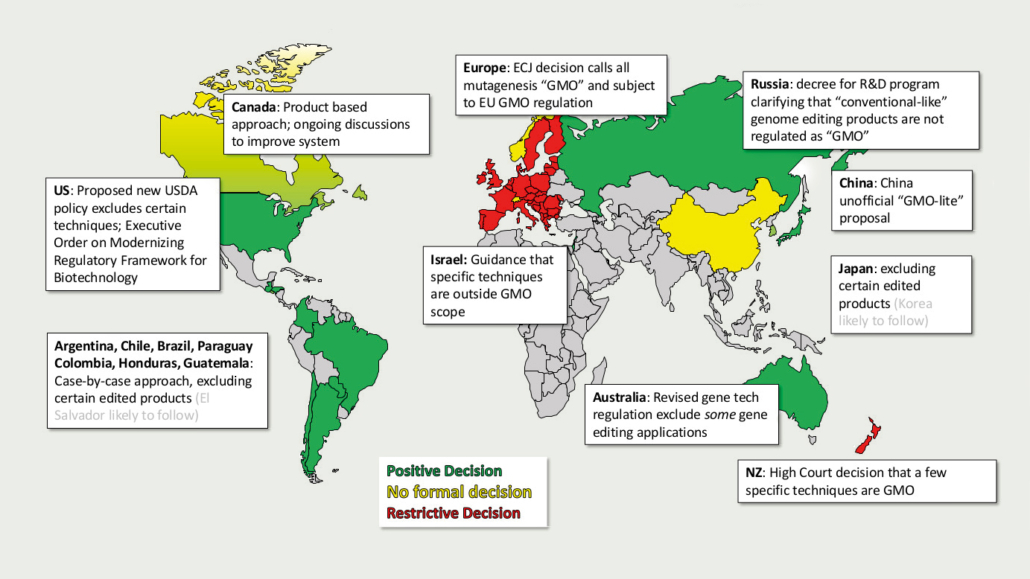
After years of regulatory deadlock concerning political decision making on genome-edited and genetically targeted mutational breeding, the European Council has demanded that the European Commission regulate new plant breeding techniques (NBT), such as oligonucleotid-directed mutagenesis (ODM), gene scissors (CRISPR), and others.
Just two weeks before the new European Commission’s head, Ursula von der Leyen, was set to be confirmed by election, the European Council demanded the Commission to launch a new study investigating how to regulate genome-edited plants in the EU. Major EU importers, such Australia, the US, Canada, and South American states Brazil and Argentina, have deregulated genome-edited crops. Now, the Council said the national competent authorities face practical problems in implementing a legal interpretation of the European Court of Justice (ECJ) ruling. Back in 2018, the EJC ruled that targeted mutagenesis falls under the strict EU safety and labelling rules for GMOs, whereas non-genetically mutagenesis methods were exempted. Currently, there is no reliable scientific method available that can distinguish crops that were generated by targeted mutation from crops that were randomly mutagenised by radiation or chemicals. Thus the Council wants the Commission to clarify through an investigation how novel genomic mutagenesis methods are to be legally classified and regulated in the future. The results of this study are to be presented by April 2021 – including legal proposals and an impact assessment.
Member states pro deregulation
The initiative was driven by the Netherlands and supported by Spain, the UK, and Scandinavian countries that want to modernise the EU GMO rules, which date back to the 1990s. Scientists and plant breeders have lobbied for a modernisation of the EU Directive 2001/18/EC because, in their view, the EU legislation is outdated and does not reflect state-of-the art, modern breeding technology. The ECJ ruling cannot be implemented, warns an open letter from 23 agricultural and food industry associations. This refers to a special feature of the new procedures: In contrast to genetically engineered plants, those produced by ODM or mutational gene knock-out through CRISPR-Cas9 cannot be reliably identified. Analytically, it is impossible to distinguish whether a mutation was triggered by genome editing, or whether it occurred accidentally and without technical intervention at a reasonable cost. As a result, the labelling obligations of EU Directive 2001/18/EC and the resulting freedom of choice cannot be guaranteed for gene-edited products. Most recently, a European citizens initiative, founded by life sciences students in July, was officially registered under the name Grow Scientific Progress. This initiative aims to collect one million signatures from EU citizens to support a liberalisation of new breeding technologies under Directive 2001/18/EC.
Industry concerns
Specifically, the action demands that the European Commission differentiate between new and old breeding methods. Furthermore, the Council proposes to define a period of time after which a specific mutation-inducing method is regarded as safe, and wants to establish a cut-off date for so-called traditional breeding. It also calls for changing the conditions under which crops that were generated by mutational methods are exempted from the GMO directive and defines such traits that are introduced by NBT, which poses a proven safety risk.
first published in European Biotechnology Winter Edition 2019


 Unsplash+
Unsplash+
