Ferring bags slipped disk pain drug
30.08.2016 - Swiss biopharma company Ferring has acquired the exclusive development and commercialisation rights to Seikagaku's Phase III chemonucleolytic leg pain treatment for €85m.
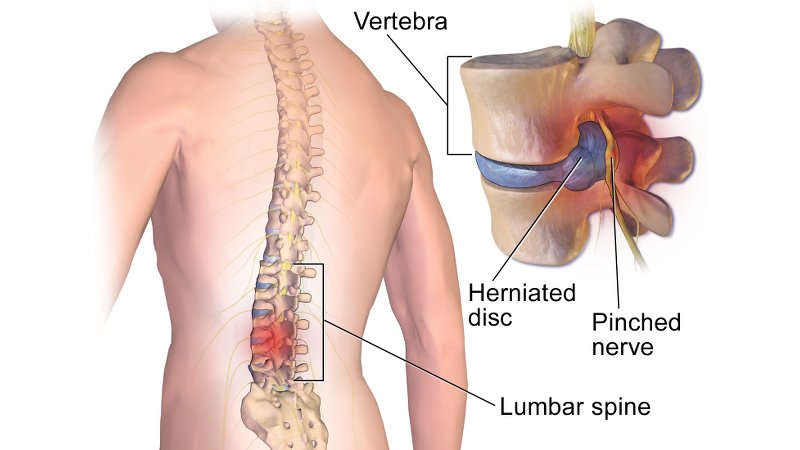
Seikagaku Corporation is granting Ferring Pharmaceuticals the exclusive worldwide rights excluding Japan for its product SI-6603. SI-6603 (condoliase) is currently being developed in two Phase III trials for for leg pain – or sciatica – due to lumbar disc herniation. Injecting condoliase into the intervertebral disc of slipped disk sufferers would degrade its main component glycosaminoglycans and cause reduction of the pressure on the nerves by shrinking the nucleus pulposus.
Under the terms of the agreement, Seikagaku is responsible for completing development and obtaining US regulatory approval. With FDA approval in hand, Ferring will commercialise the product in the US and has received further rights to develop, register and commercialise condoliase everywhere but in Japan. In return, Ferring will pay US$5m (€4.5m) upfront as well as up to US$90m (€80.6m) in development and commercialization milestones.
Condoliase could offer a non-surgical alternative to patients for whom conservative therapy and/or corticosteroid injections have failed to provide durable relief, while maintaining the option for surgery should it later become medically necessary, commendted Ray Baker, past president of the North American Spine Society.
We believe condoliase may answer a substantial unmet need among those patients suffering from radicular leg pain due to lumbar disc herniation, added Michel Pettigrew, President of the Ferring Executive Board and COO. This is a significant opportunity to expand our global Orthopaedics franchise with a new innovative drug therapy.


 Bayer AG
Bayer AG
 Picture from Ferdinand Stöhr on Unsplash
Picture from Ferdinand Stöhr on Unsplash