Shire licensed bispecific antibody from Novimmune
Swiss biopharmaceutical company Novimmune grants pharma company Shire a worldwide license to develop and commercialise a pre-clinical bispecific antibody candidate for haemophilia A.
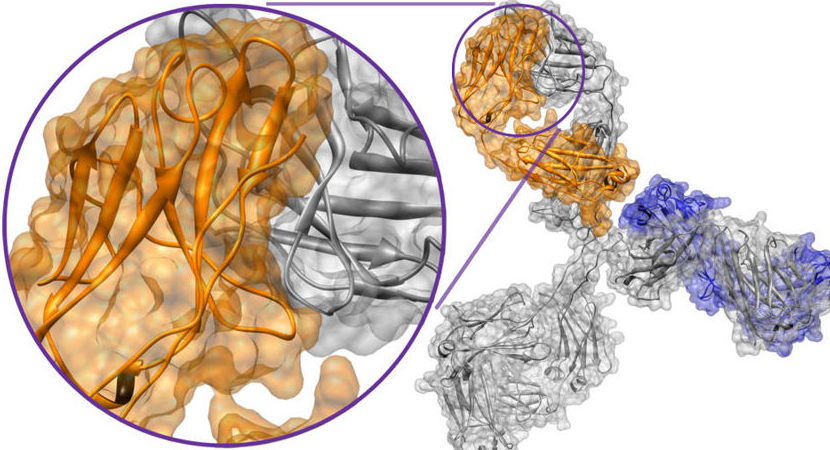
Antibody-specialist Novimmune SA and rare disease expert Shire Pharmaceuticals plc entered an agreement, granting Shire exclusive global rights to develop and commercialise a novel, bispecific antibody in pre-clinical development for the treatment of haemophilia A. The deal builds on a collaboration between privately-held Novimmune and Shire that is ongoing since 2015 to generate and evaluate Factor VIII mimetic, bispecific antibodies. According to the partners, several promising and potentially highly differentiated leads have been identified.
Financial details of the deal were not disclosed. This novel programme builds on our extensive monoclonal antibody (mAb) capabilities, as well as on our in-depth scientific expertise in haematology, said Fritz Scheiflinger, Head of Global Research at Shire. While further development and clinical trials are needed to fully evaluate this antibody, we are delighted by the potential we have seen in early discovery and the promise it may hold for haemophilia A patients. The fully-human, bispecific IgG antibody targets FIXa and FX. It is designed to imitate the body’s natural factor VIII-driven coagulation.
Shire has been steadily building its mAb research capability, which includes programmes in hereditary angiooedema (HAE), diabetic macular oedema, antibody-mediated autoimmune disease, and anti-thrombotic therapy – all of which are signatures of Shire’s new Rare Diseases Innovation Center coming to Cambridge (USA).
© www.european-biotechnology.com/hm



 SANOFI
SANOFI