MS: Gut microbobes drive inflammation
Two research team independently report they have identified specific microbes in the gut of mice and of treatment-naive patients with multiple sclerosis (MS) regulating immune responses. Phase I trails with fecal microbial transplants (FMT) are scheduled to begin early next year.
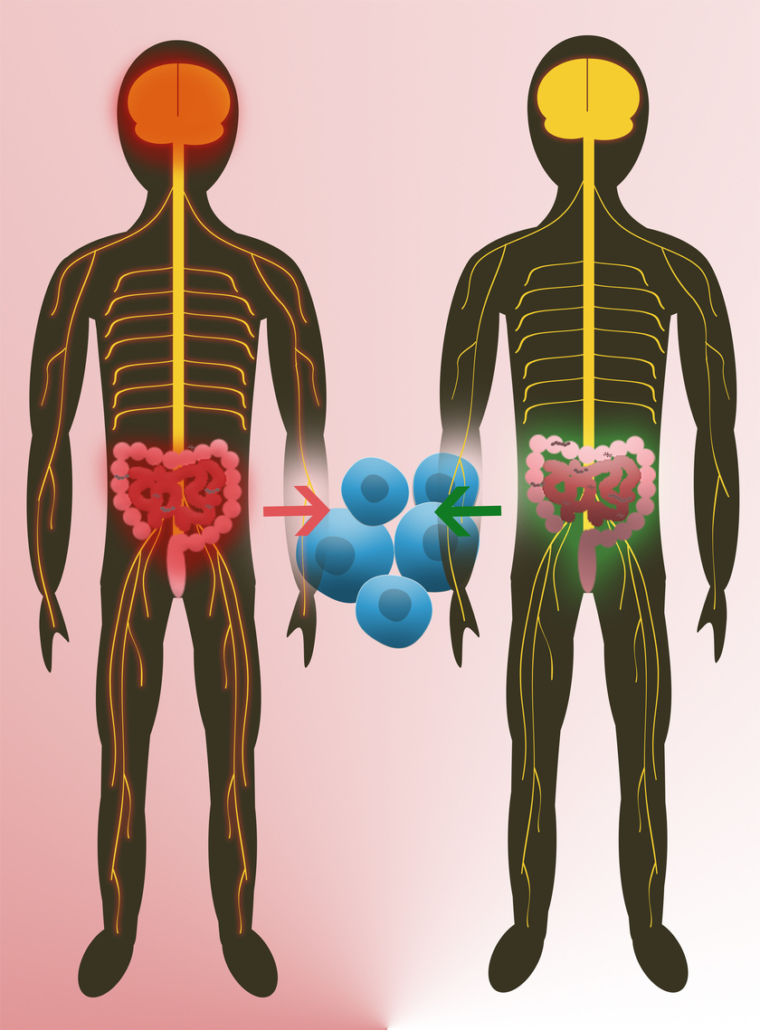
For the first time, two research groups, headed by Sergio E Baranzini from USCF (US), and Hartmut Wekerle from MPI for Neurobiology (Martinsried, Germany) and the neuroimmunologist Reinhard Hohlfeld, reported a link between certain genera of the gut microbiome with the autoaggressive inflammation that drives destruction of the myelin sheat, the insulation of neurons, in mice and patients with multiple sclerosis (PNAS, doi: 10.1073/pnas.1711235114, doi: 10.1073/pnas.1711233114). A growing number of studies had previously shown that gut microbes can directly influence the function of the human immune system, which turns against myelin in MS. Particularly, mice engineered to develop experimental autoimmune encephalitis (EAE), a model of multiple sclerosis (MS), remained disease-free when raised in a germ-free environment, suggesting that commensal microbiota mediate brain autoimmunity. Since the intestine is actually the most intimate connection between the outside world and the immune system, the researchers hypothesised that the gut microbiome could play a role in the onset or progression of MS.
To understand the role of human gut microbiota in MS, Sergio Baranzini and colleagues analysed the microbiomes of 71 MS patients and 71 healthy controls, aged 19-71, and found significant differences in the relative abundances of certain bacterial genera. Acinetobacter and Akkermansia were significantly more abundant in MS patients than in controls, whereas Parabacteroides was less abundant in patients than in controls.
Exposing human peripheral blood mononuclear cells to extracts from Acinetobacter and Akkermansia bacteria increased differentiation of proinflammatory T helper (Th) cells. Acinetobacter extracts also inhibited regulatory T cell (Treg) differentiation, whereas Parabacteroides extracts promoted Treg differentiation. Similar differences in T cell differentiation were observed in germ-free mice colonised with individual bacterial genera. According to the authors, the results could pave the way for microbiome-based therapies for autoimmune diseases.
The results of Wekerle, Hohlfeld and colleagues also point to a link between the gut microbiota and MS. His team examined 34 human identical twin pairs, aged 21-63, in which only one twin had MS. When microbiota derived from the twins were transplanted into EAE-susceptible mice, the mice receiving transplants from MS patients had a greater incidence of EAE than those receiving transplants from healthy donors.
While both results suggest that components of the human microbiome can contribute to MS, Baranzini wants to confirm the results in a larger study. We created the iMSMS (international MS microbiome study) to expand this research to other populations, and to a larger group of patients, he told European Biotechnology. At the same time his team is preparing clinical studies to check if fecal microbial transplants could become a treatment option for some of the 2.5 million MS patients, globally. The first step would be a Phase I clinical trial to ensure this is a safe procedure for people with MS, he explains. We recognize that with many available therapies in MS not every patient would be a candidate for a FMT. However, if proven safe and effective, this could be first line option for many patients. The researchers expect to start first-in-man trials early next year.
Though the number of studies using FMT are limited, they have been shown to be an effective treatment for patients suffering from Clostridium difficile infection (CDI), whose effects can range from diarrhea to pseudomembranous colitis.



 Boehringer Ingelheim
Boehringer Ingelheim Olga Yastremska, New Africa, freepik
Olga Yastremska, New Africa, freepik