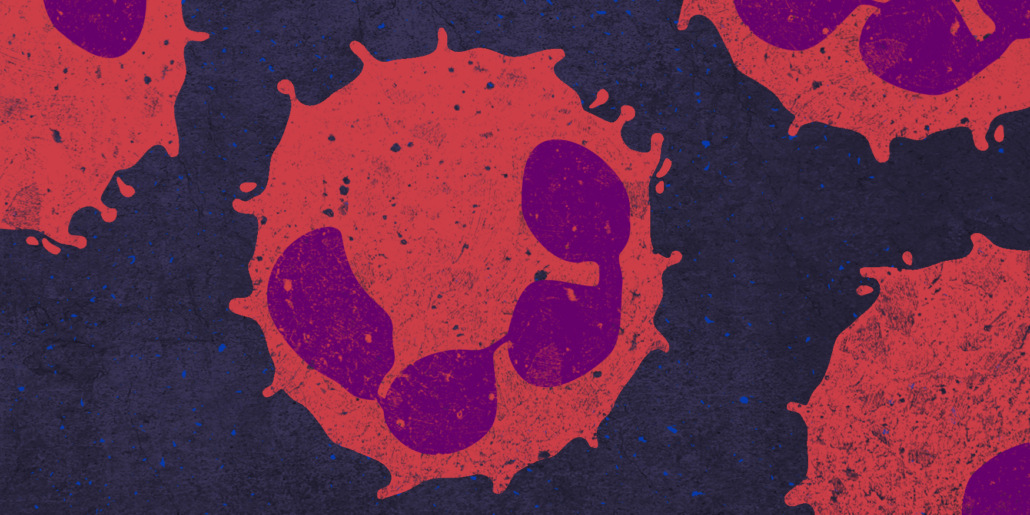
Western diet triggers inflammatory diseases
Immunologists headed by Eicke Latz (Univ. of Bonn, Germany) have found out that calorie-rich, fatty Western diet reprogrammes the innate immune system to become hyperreactive to inflammatory triggers. This could have huge medical impact as inflammation is a hallmark of most diseases of civilisation such as diabetes, cardiovascular and autoimmune disorders.
In mice that mimick atherosclerosis, the researchers from the University of Bonn and partners found that a transient 4-week calorie-rich and fatty diet with fast food (Western diet) led to a longlasting preactivation of the innate immune system. This priming of the body’s first-line defense against pathogens and exogenous irritants remained even after the animals were shifted back to their normal cereal diet (Cell, , doi: 10.1016/j.cell.2017.12.0139). Such kind of innate immune cell priming or immunologic memory had been so far observed after infections and is dubbed trained immunity. When the pathogen invades the body again trained immunity leads to a faster and stronger reaction of the innate immune system compared to the initial invasion. Now, the researchers proved for the first time that sensor molecules of the innate immune system also recognise fast food as pathogenic or dangerous".
The good news is that group leader Latz, lead author Anette Christ and co-workers were able to decipher the principal activation mechanism and how it could be potentially blocked. The unhealthy diet led to an unexpected increase in the number of certain immune cells in the blood of the mice, especially granulocytes and monocytes, said Christ adding that this provides a hint to activation of immune cell progenitors in the bone marrow.
In fact, gene expression and chromatin analysis of those immune progenitor cells revealed that the transient Western diet permanently reprogrammed the cells’ gene activity towards a proinflammatory phenotype. Its pattern resembled the type I interferone response to bacterial infections. Additionally, the monocyte and granulocyte progenitors of the mice overexpressed the proinflammatory cytokine interleukin 6 (IL-6). In cell culture experiments, the pre-primed progenitor cells showed immunological hyperreactivity after experimental exposure to antigens such as bacterial lipopolysaccharide (LPS).
But how exactly was this memory of innate immune system induced in the myeloid progenitor cells? Blocking activity of the NLRP3 inflammosome in the progenitor cells – the sensor, which normally rings the innate immune systems’ alarm bells upon invaders by sending out proinflammatory cytokines – led to complete protection from the effects of Western diet on the innate immune system. As earlier publications suggest that genetic alterations in ASC (the principal inflammosome adaptor protein) and IL-1-RAP, an endogenous inhibitor of interleukin-1 signalling are essential to trigger trained innate immunity, the researchers speculate that IL-1beta might mediate the induction of trained immunity. In fact, previous work has shown that IL-1beta inhibitors prevent death of mice challenged by experimental infection and block proliferation of immune progenitor cells. Quantitative trait locus analysis in human monocytes trained with oxidized low-density lipoprotein and stimulated with lipopolysaccharide (LPS) also suggested inflammasome-mediated trained immunity. Small molecules that block inflammosome signalling might thus help to prevent the Western diet-triggered inflammatory phenotypes, which finally might end up in accelerating the development of vascular diseases leading to stroke or heart attack or of type 2 diabetes.
However, finding specific inhibitors is not as easy as it might appear on first sight: NLRP3 inflammasome function is triggered by a wide variety of signals indicating infection, injury, cell stress or exposure to irritants. A long standing mystery in the field is how NLRP3 co-ordinates an in vivo response that is appropriate to the nature of its activating signal. The discovery that neutrophils (see photo) drive IL-1beta production in response to a subset of NLRP3 agonists provides a potential mechanism by which in vivo NLRP3 inflammasome responses can be shaped according to the type of danger encountered. On the other hand, a drug might also send the wrong signal.
Our findings have important societal relevance, stressed Latz. The foundations of a healthy diet need to become a much more prominent part of education than they are at present. Only in this way can we immunise children at an early stage against the temptations of the food industry. Children have a choice of what they eat every day. We should enable them to make conscious decisions regarding their dietary habits.



 SANOFI
SANOFI