AZ COPD drug fails in Phase III study
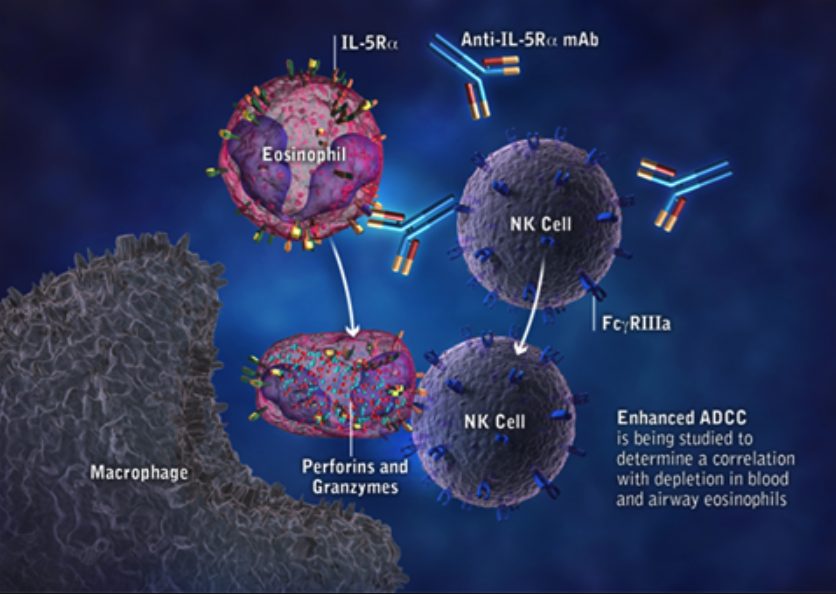
British drug maker AstraZeneca's IL-5Ralpha blocker benralizumab did not reduce exacerbations significantly compared to placebo in patients with moderate to severe chronic obstructive pulmonary disease (COPD).
After the failure in the Phase III study enroling 1556 COPD patients, hopes of the British drugmaker are turning to a second ongoing Phase III study in the indication with readout in H1/2018. In the current Phase III study, benralizumab (Fasenra) was given subcutaneously or through inhalation in addition to standard of care. COPD is a debilitating disease with significant unmet need among patients whose disease remains uncontrolled despite treatment with existing inhaled therapies. We will now await the results of TERRANOVA and a full evaluation of both trials to determine next steps for Fasenra in COPD, said Dr. Sean Bohen, Executive Vice President, Global Medicines Development and Chief Medical Officer. Fasenra is AstraZeneca’s first respiratory biologic and is currently approved as an add-on treatment for severe eosinophilic asthma in the US, EU, Japan and several other countries.
It’s not the first time Fasenra has failed to improve COPD exacerbation rates. Back in 2014, the drug missed its primary endpoint in a Phase IIa study. In the 101-patient trial, Fasenra fell short against placebo at holding off episodes when symptoms suddenly worsen. at holding off episodes when symptoms suddenly worsen.
GlaxoSmithKline, which markets the first-in-class anti-IL-5 antibody mepolizumab has filed a BLA in COPD after the drug reduced the exacerbation-rate , which is driven by the proinflammatory effect of eosinophiles, which are reduced by the drug.


 Bayer AG
Bayer AG
 Picture from Ferdinand Stöhr on Unsplash
Picture from Ferdinand Stöhr on Unsplash