Roche set to extend Hemlibra label
The US FDA has accepted a supplemental BLA to Roche's existing market approval of the new hemophilia A treatment Hemlibra (emicizumab), which could significantly extend the patient group covered by the label.
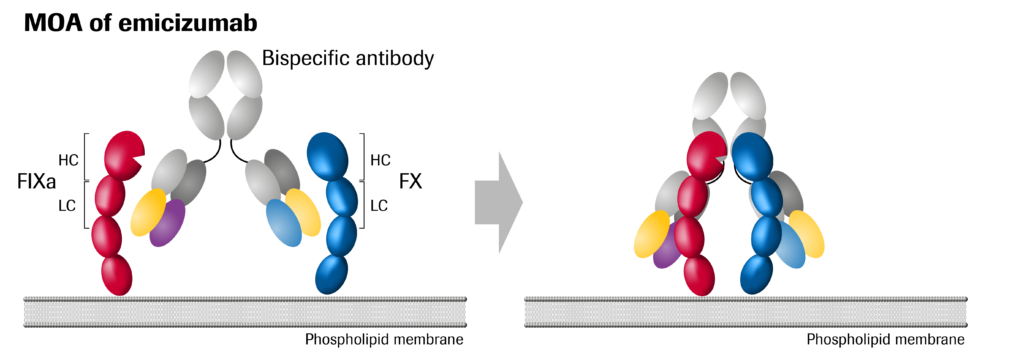
In December 2017, the FDA approved Roche’s bispecific antibody for the about 30% of 320,000 hemophilia A patients worldwide who have antibodies against blood clotting factor VIII in their blood and thus do not benefit from the current standard of care: preventive injections of blood clotting factor VIII their body is not able to form. Hemlibra is a bispecific antibody that is not affected by factor VIII antibodies, but at the same time takes on the job of factor VIII to bring together factor IXa and factor X, proteins required to activate the natural coagulation cascade (in course of the process of intrinsic platelet activation) and restore the blood clotting process for people with haemophilia A.
Recently presented results of the Haven 3 Phase III study (NCT02847637) now suggest, that Hemlibra could additionally outperform current plasma-derived or recombinantly produced factor VIII medicines by reducing bleeds more effectively than the current standard strategy to prevent bleeds in hemophilia A patients. They are part of the sBLA submitted by Roche to the FDA, the EMA and other regulatory authorities across the world. If it went through, Roche’s medicine could become the first-line treatment option for the about 70% of hemophilia patients who do not form factor VIII inhibitors. The FDA has granted Priority Review to check the new data submitted and the appropriate label extension. A decision is due to be made on 4 October.
In the HAVEN 3 study, 152 patients hemophila A patients older than 12 years without factor VIII inhibitors who received Hemlibra prophylaxis every week or every two weeks showed a 96% and 97% reduction in treated bleeds, respectively, compared to those who received no prophylaxis. In an additional arm of the study, people who had previously received factor VIII prophylaxis in a non-interventional study switched to Hemlibra prophylaxis, allowing for an intra-patient comparison of two prophylaxis regimens. Based on the intra-patient comparison, Hemlibra demonstrated a statistically significant reduction of 68% (p<0.0001) in treated bleeds, making it the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the standard of care.
There were no unexpected or serious adverse events (SAEs) related to Hemlibra in the HAVEN 3 study, and the most common AEs were consistent with previous studies, which included thrombotic microangiopathy and throboembolism as SAE. Five deaths, which occurred in previous clinical tests were not associated with Hemlibra association, the company said.
People with haemophilia A can face significant challenges in managing their condition and may need to adapt their daily lives to avoid bleeds and accommodate treatment, commented Sandra Horning, MD, Roche’s Chief Medical Officer and Head of Global Product Development. We believe the FDA’s decision to grant Priority Review to Hemlibra underscores its potential to improve the standard of care for people without factor VIII inhibitors and to help reduce treatment burden by offering more flexible subcutaneous dosing options. We look forward to working with the FDA to hopefully bring Hemlibra to all people with haemophilia A as quickly as possible.
Analysts previously prognosed Hemlibra to achieve peak sales of US$4bn annually in the growing US$15.8bn hemophilia A market, which is currently transformed by major acquisitions driven forward by Sanofi and Takeda.



 Boehringer Ingelheim
Boehringer Ingelheim Olga Yastremska, New Africa, freepik
Olga Yastremska, New Africa, freepik